af Sara Cordoba 4 år siden
344
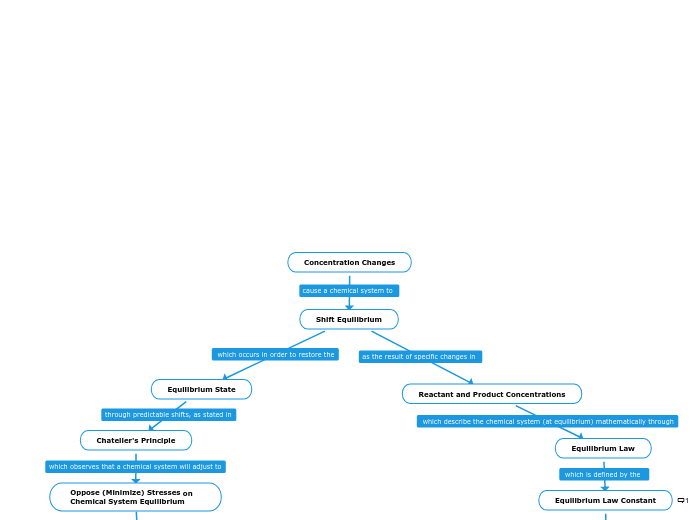
Concentration Changes
af Sara Cordoba 4 år siden
344

Mere som dette
Equilibrium Law Constant
Remains constant during changes in Concentration
Position of the Equilibrium
Change Concentration of Entities in Equilibrium
Increase in Reactants/Decrease in Products Concentration
Reactants > Products
Forward Reaction (Reduce [Reactant])
Products Concentration Increase as Reactants Concentration Decrease
Product Production ⇀
[Product] Decrease
Collision Theory
Increase Particle Collision Frequency (Probability of Successful Collisions)
Decomposition into Reactants
[Products] must be Used Up
Equilibrium Graphs
Formation (Concentration) of Products
[Reactants] must be Used Up
Increase in [Products]/ Decrease in [Reactants]
Reactants < Products
Reverse Reaction (Reduce [Product])
Reactants Form as Products Concentration Decrease
Reactant Production ↽
[Reactant] Decrease
Oppose (Minimize) Stresses on Chemical System Equilibrium