af Derelyn Higa 1 år siden
180
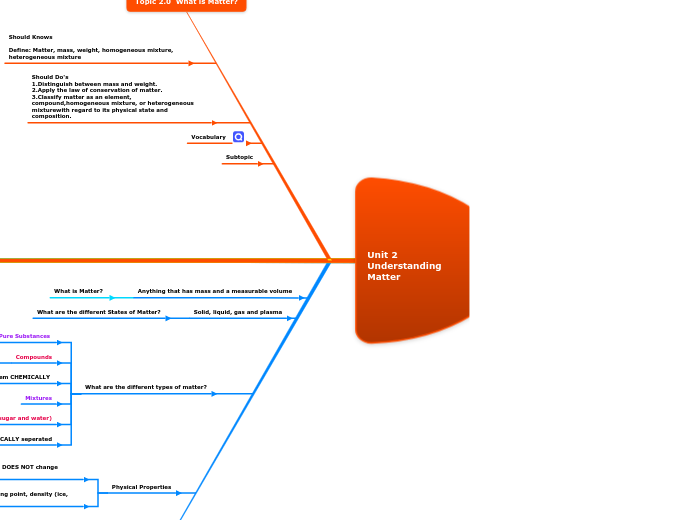
Unit 2 Ūnderstanding Matter 2024
af Derelyn Higa 1 år siden
180

Mere som dette
Heterogeneous (oil and water)
Elements