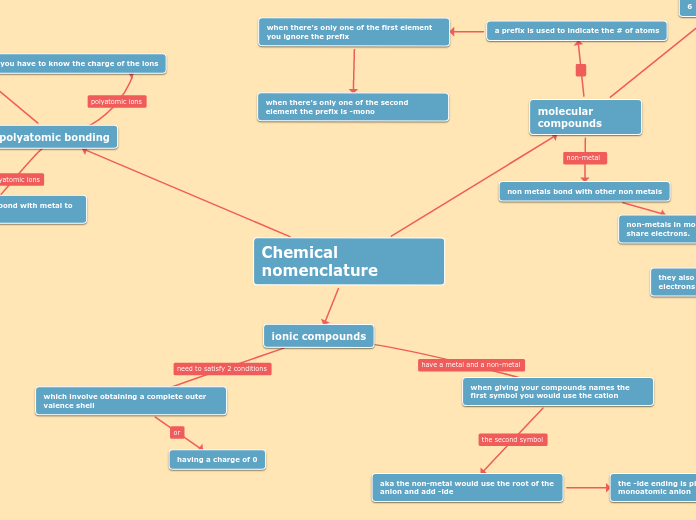
Chemical nomenclature
ionic compounds
which involve obtaining a complete outer valence shell
having a charge of 0
when giving your compounds names the first symbol you would use the cation
aka the non-metal would use the root of the anion and add -ide
the -ide ending is placed at the end of a monoatomic anion
polyatomic bonding
you have to know the charge of the ions
also known as a radical bond with metal to form ionic compounds
polyatomic anion contain oxygen
when there's 2 oxyanions formed in one element the one with more oxygen the name ends in -ate and the one with less oxygen ends with -ite
molecular compounds
non metals bond with other non metals
non-metals in molecular compounds have to share electrons.
they also create a covalent bond when electrons are shared
names of covalent bond
single
double
triple
a prefix is used to indicate the # of atoms
when there's only one of the first element you ignore the prefix
when there's only one of the second element the prefix is -mono
number of electrons shared
2
4
6