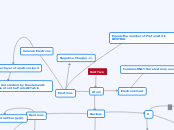
Unit Two
Atom
Nucleus
Determines if an element
is an isotope or not
Is an isotope if the mass of protons and neutrons are the same. Also is a proton if protons are the same and neutrons are not.
It is NOT an isotope if the protons,
ALONE, are not the same.
Neutrons
Mass-Protons
A
Atomic Number
Electrons
Valence Electrons
Outer layer of electron cloud
Can be determined by the
elements placement on
the Periodic Table