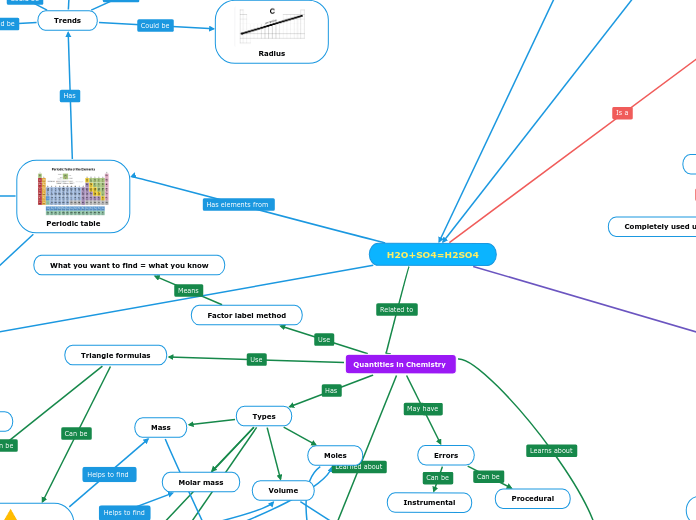
H2O+SO4=H2SO4
Periodic table
Compounds
Molecular
Polar
Non-polar
VSPER
Molecular geometry
Electron geometry
Ionic
Trends
Affinity
Reactivity of metals
Electronegativity
Ionization energy
Radius
Elements
Metals
Non-metals
Atoms
6 Theories
1. Greek
2. Dalton
3. Thompson
4. Nuclear
5. Bohr
6. James Chadwick
Ions
Cations
Positive
Anions
Negative
Nucleus
Protons
Neutrons
Orbitals
Electrons
Charge
Neutral
Molecules
Forces
Intermolecular
London Dispersion
Dipole-dipole
hydrogen bonding
Intramolecular
Diagrams
Lewis Dot
Bohr-Rutherford
Chemical reactions
Types
Synthesis
Neutralization
decomposition
Combustion
Single displacement
Double displacement
States of matter
Solid
Gas
Liquid
aqueous
Word
Water+Sulfate=Sulfuric acid
Chemical
Balances
Reactant
Limiting
Completely used up during reaction
Excess
Leftover During reaction
Products
Quantities in Chemistry
Errors
Instrumental
Procedural
Factor label method
What you want to find = what you know
Triangle formulas
Units
Gramm
Mol
Litre
metrix prefixes
Scale
Types
Mass
Molar mass
Moles
Concentration
Ways to express
Molar
Percent by volume
percent by mass
Mass per volume
Volume
Avogadro’s number
6.02*10^23
Stoichiometry
The study of quantitative relationships in chemical reactions
Solutions and gases
Solubility
PH scale
Bases
Substances that take proton from another compound
Acids
Substances that donate a proton to another compound