History of Thermodynamics
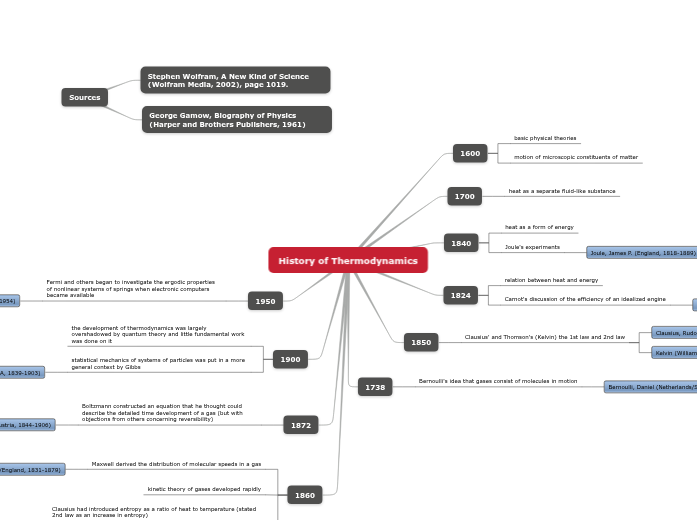
1600
basic physical theories
motion of microscopic constituents of matter
1700
heat as a separate fluid-like substance
1840
heat as a form of energy
Joule's experiments
Joule, James P. (England, 1818-1889)
1824
relation between heat and energy
Carnot's discussion of the efficiency of an idealized engine
Carnot, Sadi N. L. (France, 1796-1832)
1850
Clausius' and Thomson's (Kelvin) the 1st law and 2nd law
Clausius, Rudolf J. E. (Germany, 1822-1888)
Kelvin (William Thomson) (Scotland, 1824-1907)
1738
Bernoulli's idea that gases consist of molecules in motion
Bernoulli, Daniel (Netherlands/Switzerland, 1700-1782)
1950
Fermi and others began to investigate the ergodic properties of nonlinear systems of springs when electronic computers became available
Fermi, Enrico (Italy/USA, 1901-1954)
1900
the development of thermodynamics was largely overshadowed by quantum theory and little fundamental work was done on it
statistical mechanics of systems of particles was put in a more general context by Gibbs
Gibbs, J. Willard (USA, 1839-1903)
1872
Boltzmann constructed an equation that he thought could describe the detailed time development of a gas (but with objections from others concerning reversibility)
Boltzmann, Ludwig E. (Austria, 1844-1906)
1860
Maxwell derived the distribution of molecular speeds in a gas
Maxwell, James Clerk (Scotland/England, 1831-1879)
kinetic theory of gases developed rapidly
Clausius had introduced entropy as a ratio of heat to temperature (stated 2nd law as an increase in entropy)