Ionic Compounds: a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ions called anions. Bibliography: Lewis structure. (2017). En.wikipedia.org. Retrieved 21 February 2017, from https://en.wikipedia.org/wiki/Lewis_structure
Bohr's Model
Explenation
In Bohr's Model, atoms are depicted as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus. The transfer of atoms is depicted as the outer electrons of one atom passing to the other.
Example

Salt (Sodium Chloride), NaCl
Bibliography
Bohr model. (2017). En.wikipedia.org. Retrieved 21 February 2017, from https://en.wikipedia.org/wiki/Bohr_model
Cite a Website - Cite This For Me. (2017). W3.shorecrest.org. Retrieved 21 February 2017, from http://w3.shorecrest.org/~Lisa_Peck/Physics/syllabus/matterproperties/images_webpage/ionic.gif
Lewis Dot Diagram
Explenation
In Lewis Dot Diagram, atoms are represented with their chemical symbol with each dot around it being an outer electron. The transfer of electrons is representad as the dots traveling from one atom, to the other.
Example
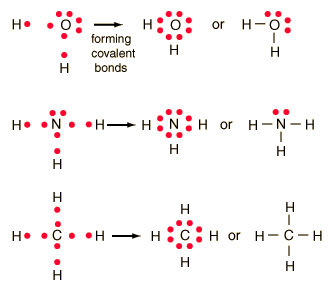
1. Water (Hydrogen Oxide), H2O 2. Ammonia (Nitrogen Hydride), NH3 3. Methane (Carbon Hydride), CH4
Bibliography
Cite a Website - Cite This For Me. (2017). Hyperphysics.phy-astr.gsu.edu. Retrieved 21 February 2017, from http://hyperphysics.phy-astr.gsu.edu/hbase/Chemical/imgche/Lewisbond3.gif
Ion Method
Explenation
On the Ion Method, atoms are depicted with there symbol and labeled either positive (if they have more electrons than necessary) or negative (they lack the electrons to be stable). The transfer of atoms is depicted with the value so each crossing, and then placed on the final compound formula.
Example
Salt (Sodium Chloride), NaCl
Bibliography
Cite a Website - Cite This For Me. (2017). Chem.libretexts.org. Retrieved 21 February 2017, from https://chem.libretexts.org/@api/deki/files/8649/151ionic.GIF?size=bestfit&width=256&height=288&revision=1