von Anika Bradley Vor 5 Jahren
559
Mass Moles Number of Particles Assignment
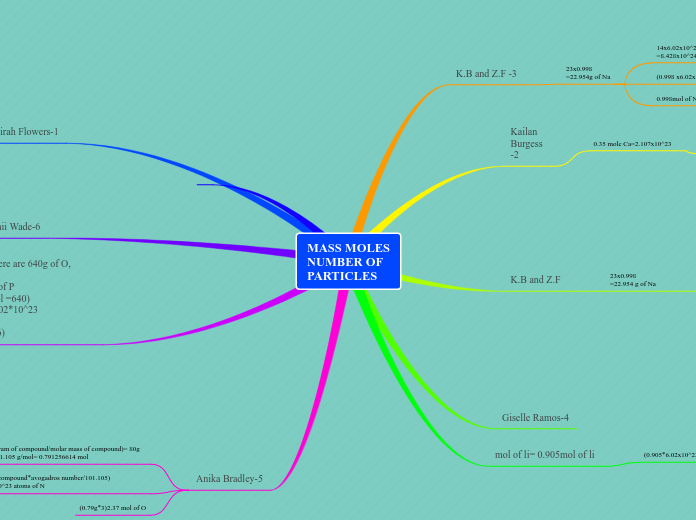
von Anika Bradley Vor 5 Jahren
559

Mehr dazu
0.998x23 =22.954 mole of Na