Climate Change

Lesson #3 - Past Climates
People have been studying, recording and analyzing the weather for only a few centuries. But, in order to understand what was the climate like a few centuries ago, scientists have to get creative. Paleoclimatologists are people who study and analyze the past climates.
Ways to analyze past climates

Tree rings
Temperature and rainfall both have an effect on tree growth. The total amount that a tree grows during each season is determined by the size and the color for each annual ring. Dendrochronologists study and analyze the details about the size, color, and the shape of each ring. A wide ring indicates wet and cool weather, which allows trees to grow faster. A thin ring, however, indicates that it was grown in dry and hot conditions, when tree growth is a lot slower. A dark ring indicates the growth during the late summer season, and a light-colored ring means the tree had been grown during the springtime.

Ice cores
Much like tree rings, the layers of snow and ice gather together each year. In order to show the evidence of past climate conditions, scientists use a specific drill designed to drill deep through the layers to extrect long, cylinder-shaped samples which are called ice cores. Scientists then analyze the thickness and the composition of ice in each layer to make speculations about the past climates.
Evidence of Past Climates Obtained from Ice Cores
Dissolved and particulate matter in ice
(Dust, ashes, salts, plant pollen and other materials)
The frozen samples of these materials gives clues about previous events and conditions, such as volcanic eruptions, meteorite impacts, and wildfires.
Physical characteristics of the ice
(crystal size and shape)
Characteristics help identify the conditions of temperature and humidity around the time the ice crystals were formed.
The composition of the trapped air bubbles
When water freezes, the tiny air bubbles in the water becomes trapped in the ice. The air pockets allow scientists to study and analyze the greenhouse gases concentration over centuries and thousands of years.
The composition of the ice
Water consists of differing proportions of hydrogen and oxygen isotopes. Isotopes are different variants of an atom. Water that consists of oxygen-18 freezes at a much higher temperature than oxygen-16 water. Furthermore, water that consists of oxygen-16 tend to evaporate much quicker than oxygen-18 water. For that reason, the concentration of isotopes in different layers of ice represents the temperature around the time the ice had formed.

Fossils
The remains or traces of living things, which are known as fossils, also provide clues when paleoclimatologists analyze and recreate the past climates. The many types and plentifulness of fossilized remains in each rock layer can help scientists recreate the environment around the time the layer formed, including the climate. Since plants and animals are differently adapted in the environment to where they live, analyzing the fossils can help determine about what the environments looked like millions of years ago.
The Rate of Climate Change
Scientists are stil discussing whether climate change is affected by gradual or by catastrophic changes. But, this question is at the center of a disagreement about whether human activity is the main cause of climate change. However, many climatologists agree that humans are affecting the structure of Earth's atmosphere. This affection may increase or decrease the rate of the climate change progression.
Evidence of Past Climates from Sedimentary Rock
In order to get evidence of climates which are older than can be analyzed in ice cores (1 million+ years old), scientists have to examine and analyze rocks. Every year, billons of tons of sediments (rock fragments) wash from the land and gather together in thick layers on ocean floors and lake beds. The hard parts of microscopic sea creatures, like algae and diatoms, as well as pollen from flowers are preserved in these sediments. Over long periods of time, the deposited material becomes pressured and hardened to later form into sedimentary rock.
EPICA and GRIP
The European Project for Ice Coring in Antarctica (EPICA) had gathered climate data from around 800,000 years ago.
The drilling had reached 3270 meters deep.
The data is then compared with research from the Greenland Ice Core Project (GRIP).
As a result, the data lead to a more complete picture and offered a better understanding of climate change.

Lesson #2 - The Greenhouse Effect

Greta Thunburg - a teenager who wants the world to know about the consequences of global warming
The greenhouse effect is a natural process that has been occuring for millions of years. The gases and clouds take in infrared radiation that has been emitted from Earth's surface and radiate it in all directions, heating up the atmosphere as well as the surface.
The climate system moderates Earth's temperature by absorbing and storing energy from the sun and distributes it worldwide. So for that reason, every day and night, the temperature always remain constant.
Without the greenhouse effect, which is needed for life to exist and thrive, the earth's temperature would be 255 Kelvin (-18℃) instead of 15℃ (Earth's average temperature)
Greenhouse Gases
Greenhouse gases are any gases in the atmosphere that absorbs lower energy and infrared radiation. The majority of the air is made up of nitrogen and oxygen, which do not absorb the radiation from the surface. Greenhouse gases are trapped in the troposphere, which is up to 16,000 meters above the Earth's surface.
Carbon dioxide
Carbon dioxide (CO2) makes up about 385 ppm of the Earth's surface, which represents exactly about 0.0385%. However, CO2 is expected to cause up to a quarter of the natural greenhouse gases on Earth. CO2 molecules also have atoms that vibrate and wiggle in different ways, but can also absorb different types of energy.
Anthropogenic (Human Influenced) Sources of Carbon Dioxide
Burning fossil fuels (coal, gasoline, natural gases), releases CO2
Deforestation - restrains photosynthesis, which would remove CO2 from the atmosphere; as leftover forest waste decomposes, it becomes greenhouse gases
Carbon Dioxide & Global Temperature
Increasing of CO2 levels result to increasing global temperatures due to the greenhouse effect; however, increasing of the temperature can result CO2 levels to grow
As the temperature increases, the CO2 kept in plants and oceans get released

Methane
In the atmosphere, there is fewer methane (CH4) than CO2, however, it's very strong and powerful. CH4 can absorb and trap 23 times more thermal energy than CO2. It also comes from natural and human sources, and is emitted into the air from plant decompostion and animal digestion.
Anthropogenic (Human Influenced) Sources of Methane
Agriculture - rice farming, cattle ranching, etc.
Landfill/sewage treatment plants - methane is released into the atmosphere as an organic material decay
Coal mining and natural gas extractions cause methane to be released into the atmosphere
Nitrous oxide
Nitrous oxide (N2O) is 300 times more stronger than a CO2 molecule as a greenhouse gas, but it has lower concentration. N2O also comes from bacteria that produce it, as well as human sources.
Anthropogenic (Human Influenced) Sources of Nitrous Oxide
Used for nitrogen fertilizers
Fossil fuel combustion
Livestock manure
Chloroflurocarbons (CFCs)
no natural sources of CFCs
leaks out of refrigerators/air conditioners
Water vapour
2/3 of the natural greenhouse effect is caused from the water vapour in the atmosphere. The amount of water in the atmosphere can be determined by the temperature, which is about 4%. Water vapour and temperature are both associated by a positive feedback loop.
For hundreds and thousands of years, greenhouse gases have been an important part of our atmosphere and have also been involved in the natural greenhouse effect.
Positive Feedback Loop
A produces more of B, which in turn produces more of A.
For example: High temperatures causes more water to evaporate into the air and form water vapour.
Water vapour is effective on trapping heat, so that more of the vapour increases the temperature.
Negative Feedback Loop
A self-regulating system that functions in order to get more stable.
For example, in this scenario, the temperature in a heated room reaches a specific limit; the heating is then turned off, and the temperature begins to fall as a result. When the temperature drops down to the lowest limit, the heating is then switched back again.
Anthropogenic Greenhouse Effect
The growing amount of the lower-energy infrared radiation trapped from the atmosphere as a result of rising greenhouse gas levels in the atmosphere due to human activity, which is eventually causing Earth's temperature to grow warmer substantially
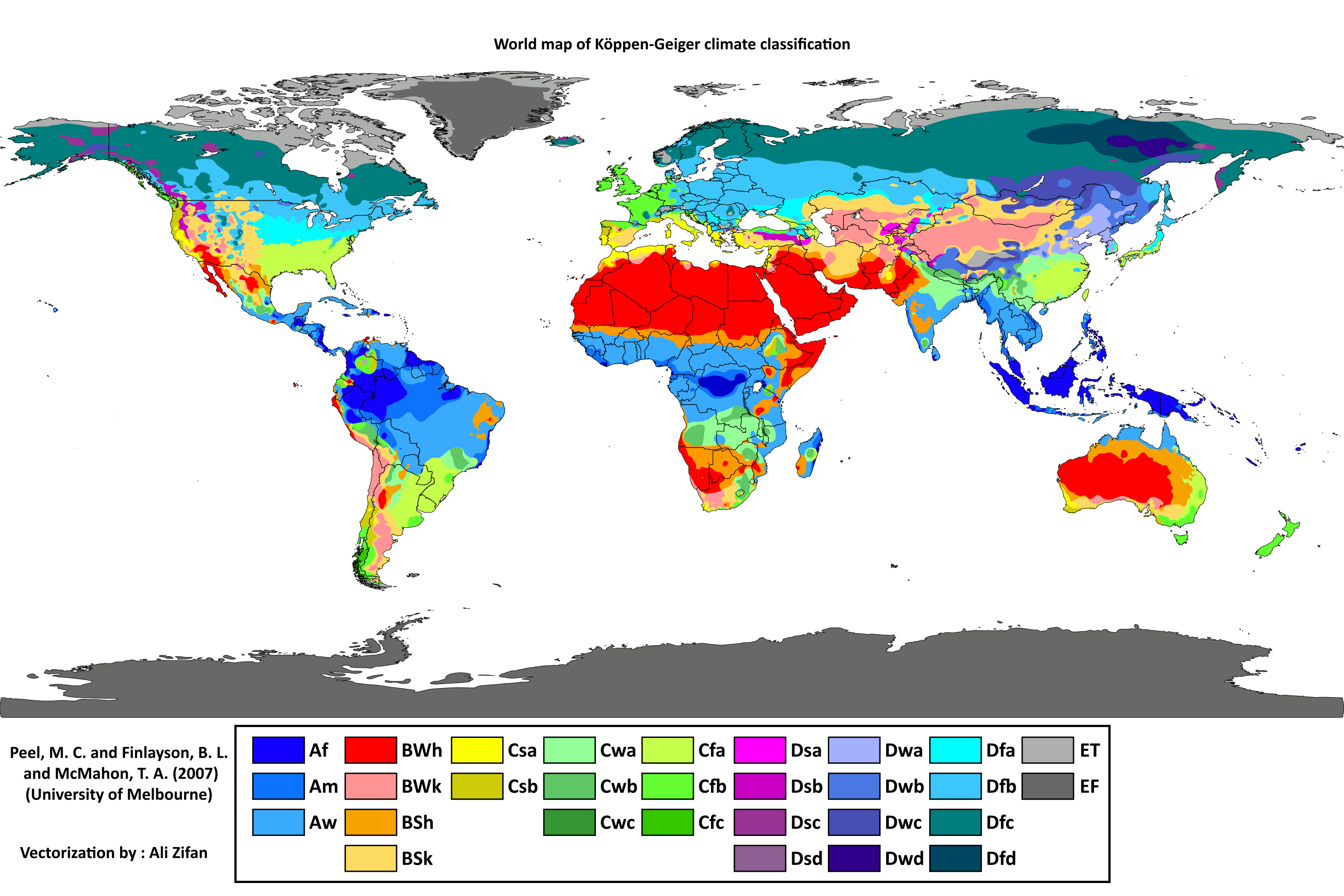
Lesson #1 - Weather & Climate
Koppen-Geiger Classification Categories
Tropical
Dry
Moderate
Continental
Polar
Factors that Influence Weather
The Water Cycle
Air Masses
Jet Streams
Weather Fronts
Climate - the pattern of the weather in a specific region over a long period of time, but also determines what plants and animals live there
Weather - conditions in a specific location that usually lasts over a short period of time
Factors Affecting Climate
Distance from equator (latitude)
Presence of large bodies of water
Presence of ocean and air currents
Land formations
Height above sea level (altitude)
How can information about climate zones be used?
Climate zones can be pretty useful for gardening and farming because plants can best grow in climate conditions which are found within the native ecosystems.
Factors that Influence Climate
Elevation or Altitude affect climate
As altitude increases, climate conditions usually become colder over time. The life zones on mountains consist of these changes, but snow has the highest elevations.
Prevailing global wind patterns
There are six main wind patterns worldwide; three in the nothern hemisphere and the other three in the south. As the seasons change over time, the wind patterns either shift northwards or southwards.
Topography
Topography can also change the climate depending on the specific location. For instance, mountain ranges can be barriers for air movement. In the Costal Range, the mountains allow for some condensation as well as light precipitation.
Effects of Geography
The specific area of where a town or city is placed, and how far it is located from mountains and water areas can help control the wind patterns and the air masses affecting it. For instance, costal areas can enjoy refreshing breezes during the summer, while cooler ocean air moves across the oceans.
Surface of the Earth
The amount of sunlight Earth's surface recieves determines how much atmospheric heating occurs. Darker areas, such as heavily vegetated regions, tend to be as good absorbers, while lighter areas, such as snow or ice-covered regions, tend to be as good reflectors. The oceans slowly absorbs and loses more heat than land. The waters then release the heat into the atmosphere, which is then carried out worldwide.
Climate change over time
Over the years, cold and warm periods have impacted Earth's history. While some had lasted over a short period of time, others had lasted for hundreds or even thousands of years. During cold periods, glaciers grew a lot bigger and had spreaded across the oceans. And during warm periods, the ice melted. These periods had negatively affected plant and animal life. Since the beginning of the 20th century, temperatures had been constantly rising across the world. However, it's not officialy clear if global warming is being caused by human activities, such as burning fossil fuels and destroying forests.
Sun & Earth’s Climate System
The climate system consists of four compound parts that interact with each other to produce Earth's climate. These parts include:

Lithosphere
Consists of Earth's rocky crust, and land surfaces

Hydrosphere
Consists of liquid water, ice, and water vapour

Atmosphere
Consists of the gases surrounding Earth

Biosphere
Consists of plants, animals, microbes or any other living thing
The climate system is powered by the sun. The energy in which the Earth recieves from the sun interacts with the climate system parts to produce climate zones.
Weather Map Key

High Pressure
A specific area of high pressure in a clockwise rotation in the northern hemisphere; counterclockwise in the south.

Low Pressure
A specific area of low pressure in a counterclockwise rotation in the northern hemisphere; clockwise in the south.

Tropical Storm
A moving cyclone with one or more locked isobars, and has a minute max of sustained surface winds of around 39 to 73 mph.

Hurricane
A moving cyclone with interlocked contours, which have a strong rotation, and can have a minute max of sustained surface winds of 74 mph and up.

Cold Front
An edge of a colder air mass, which divides the two air masses and maximizes the gradients of temperature and moisture

Warm Front
An edge of a warmer air mass, which divides the two air masses. Moisture and temperature gradients are maximized in the frontal zone.
Earth Absorbs Energy From the Sun
When radiation contacts a matter particle, one of these three things happens.
The particle may gain energy by absorbing the radiation.
The radiation may be transmitted through the particle.
The radiation my be reflected off the particle.
Earth’s Surface Emits Energy
As Earth's surface warms up from the sun's energy, it obtains thrmal energy and then converts it into low-energy infrared radiation.
The amount of energy radiated by Earth's system is equivalent to the amount of energy Earth's system absorbs from the sun.
The global temperature stays relatively constant, since this energy is balanced.
Equilibrium
The balance between the energy absorbed from the sun and the energy released from the Earth makes sure that the global temperature remains relatively constant.
If there's no climate system, the Earth would still have an energy equilibrium, but Earth would also be colder.
The greenhouse effect is also responsible for Earth not being cold. Without the greenhouse effect, which is needed for life to exist and thrive, the earth's temperature would be 255 Kelvin (-18℃) instead of 15℃ (Earth's average temperature).

Latitude & Climate Zones
The Sun's energy is more high near the Earth's equator, since it hits the surface directly
The sun's energy is low near the two poles, since the energy hits the surface at a specific area and spreads over a large area

Lesson #4 - Indicators of Climate Change

Heat Waves - For thousands of years, Earth had experienced severe weather conditions, but they are becoming more frequent, widespread, and a lot more severe than in the past. When this happens, air conditioners are turned on, resulting in a growing usage in electricity, and releasing greenhouse gases into the atmosphere. As the air gets warmer, the soil, lakes and rivers also get warmer. The climatic zone borders can shift.

Drought - Droughts are the most severe when they affect specific regions located near deserts. However, recently, there has been a lot of seasonal rain that provided water needed to grow crops and to keep animals alive in many of those regions. Warm temperatures can increase the evapration from soil, making periods with low precipitation drier than they would be in much cooler weather conditions. Droughts can also continue through a positive feedback loop, where the dry soils and decreased plant covers can put an end to rainfall in dry areas. Changing climates can also change atmospheric rivers (thin moisture streams carried in the atmosphere), which can eventually disrupt precipitation patterns in the western US.
/GettyImages-605383007-5728164c3df78ced1f3a2015.jpg)
Storms - Changes in the frequencies and the severity of storms are one potential effect of the faster increase in the average global temperature and the movement of energy worldwide.
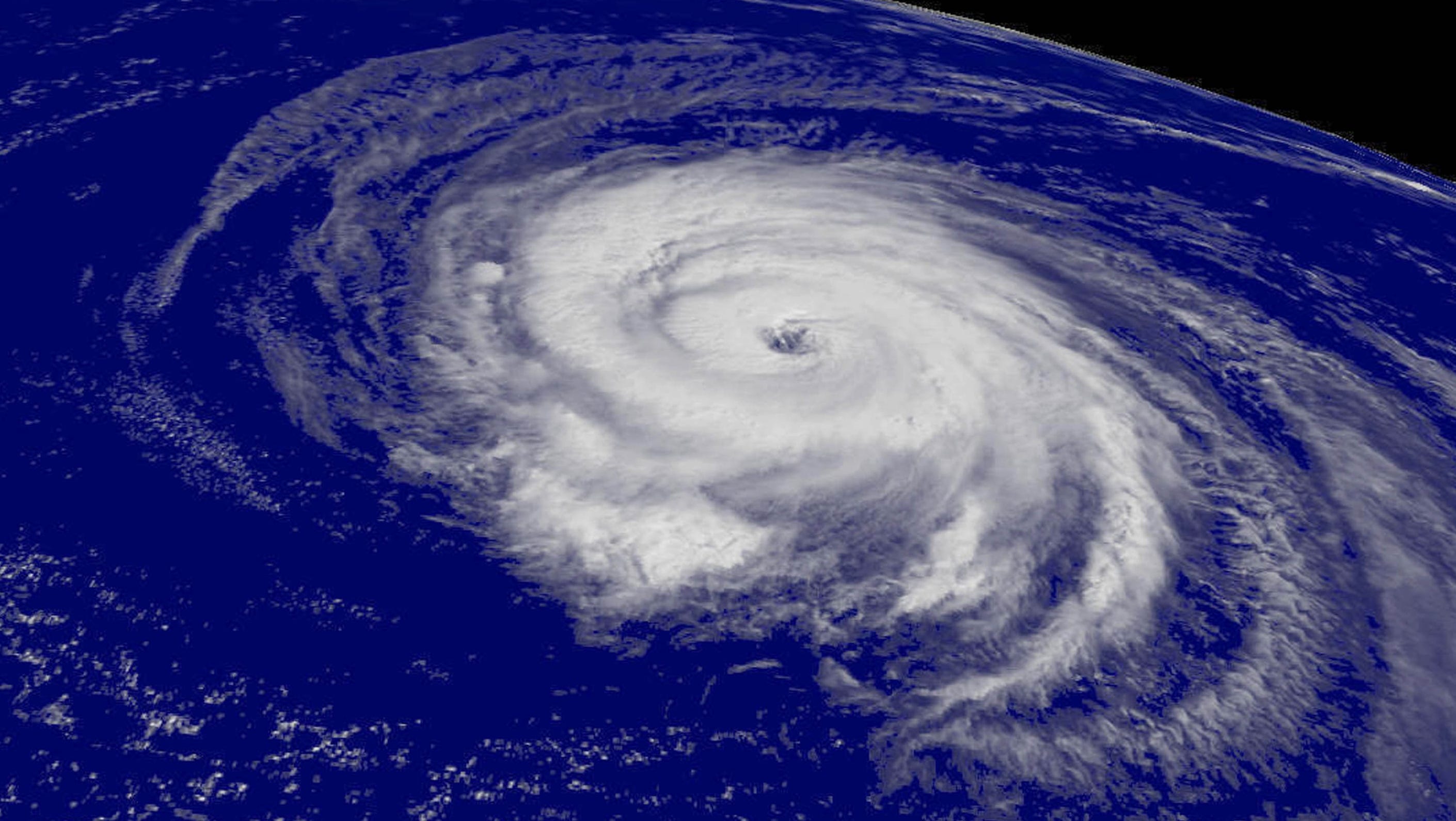
Hurricanes - Warmer sea temperatures could increase tropical storm wind speeds, possibly causing more damage when they reach contact with land. Based on categorization and classification, Category 4 and 5 hurricanes are more likely to increase, with wind speeds increasing up to 10%. Areas which are affected by hurricanes are shifting polewards. This is most likely related with expanding tropics due to higher global average temperatures.

Wildfires - When the weather conditions is hot and dry over a long period of time, the trees begin to dry, and that they start to lose their leaves as a result. The likelihood of fire begins to grow. However, even though the wildfire frequency is generally low worldwide, compared to other natural disaters such as droughts, it's still increasing. Climate change caused by human activity has had an impact on Australia's destructive wildifires that had recently occured, making the risky conditions which caused widespread burning at least 30% more likely than a world without global warming. Wildfires are also a worldwide phenomenon not only affected by heat and precipitation, but also by wind, humidity or other factors. Researchers had looked at the influences of climate change using a measurement standard, which is known as the Fire Weather Index, which takes all of the factors to help determine the risk and chances of a wildfire at a specific area in a specific time.

Floods - When the air temperature quickly becomes warm in the spring, snow can melt very rapidly for rivers and streams to handle the run-off. These seasonal floods damage residential homes, buildings, and even cropland, as they are becoming more common in certain areas across the world.

Ocean Warming - Convection ocean currents combine the cold and warm waters together. Last century, the oceans' tempratures had increased to 0.6℃, which was a bit less than the increasing air temperatures around that time. However, there are a number of reasons for worrying about the warming oceans. As the water grows warmer, it expands over time. This can eventually cause the ocean levels to rise higher, causing more costal areas at risk for being buried beneath the sea. Warmer water also absorbs less CO2 (as if cold pop absorbs more CO2 than warm pop), so it will be less effective as a carbon sink. Phytoplankton undergo the photosynthesis process and also serves as an important carbon sink. Warmer oceans kill more and more plankton over time; less CO2 is absorbed. Warm water can also create or trigger more hurricanes, which can damage costal areas and kill people.

Melting Ice - As the global temperature increases, Earth's sea and glacial ices begins to melt over time. This can eventually result in consequences other than the polar regions. Melting ice can affect the earth by changing the coastlines and altering the geographical continental coastlines, changing habitats of shoreline animals, plants and micro-organisms, causing the loss of property, reducing fresh water available for communities worldwide, and flooding the land which is above sea level. During the summertime, in the Arctic Ocean, the amount of the ice has decreased to a large extent. Over the last century, the normal leval of the oceans has increased to about 20cm. This can cause glaciers by land to melt as well as increase the water levels as it warms.
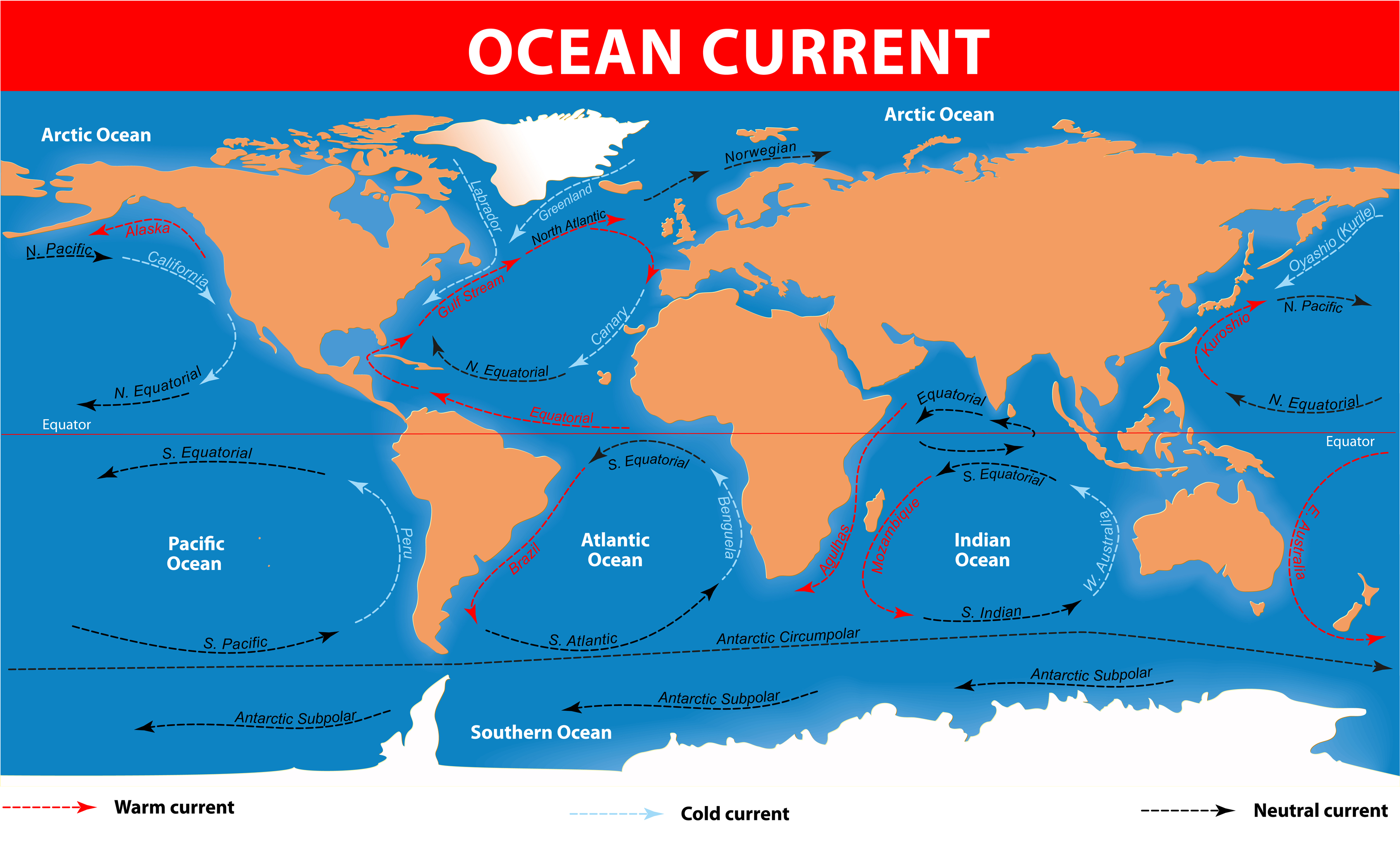
Ocean Currents - Oceans function as Earth's heating and cooling systems. As the Arctic water temperatures increases, it can lead to more extreme weather as well as devestating consequences.
Thermohaline circulation (THC) -Winds drive ocean currents up 100 meters of the oceans surface, but can also flow thousands of meters beneath the surface. The deep-ocean currents are driven by the differences in the water density, which is controlled by the temperature. Melting ice and warm waters can result in changing the flow directions of ocean currents. Ice is frozen fresh water; therefore, if the sea ice, icebergs and glaciers all melt, there will be more freshwater added to the oceans. This can eventually diminish the salt contents of the sea water. Freshwater is less dense than saltwater, so it stays on the surface. At the North Atlantic surface, the dense saltwater sinks, bringing the currents through the deep parts of Earth's oceans.

Climate Change on Wildlife - Polar bears usually walk on ice to find and catch seals, since seals swim too quick for the bears to catch them in open water. But nonetheless, if a seal finds a hole through the ice to properly breathe, the bears can catch them. Less ice causes poor hunting, and makes it more difficult for the polar bears to find their main source of food.

Affected Species - According to the IPCC, between 40-70% of all species are at risk of extinction if the global temperature reaches 3.3℃. 35% of frogs, toads, and salamanders are threatened with extinction thanks to climate change. Corals are the earliest animals, compared to jellyfish. They conceal skeletons that remains long after the animals died. As these skeletons began to build up, they eventually formed coral reefs as we know them today. Sadly, Earth had lost 20% of coral reefs thanks to warm water, sedimentation and storm damage.
Biology
Mitosis
Interphase
The cell grows to a bigger size, makes a DNA copy, and gets ready to divide into two cells. Two centrioles are also duplicated as well.

Prophase
The chromatin from the nucleus begins to condense into chromosomes. The centriole pairs then move to different sides of the nucleus. Spindle fibres begin to create a bridge between the cell ends. The nuclear envelope begins to break down.

Metaphase
The chromosomes then line up across the cell. All of them are attached to each spindle fiber as its centromere.

Anaphase
The centromeres then split into two and the two chromatids seperate from each other. One chromatid is attached to its spindle fiber while the other shifts to the opposite end. The cells stretches out while the ends are being pushed apart.

Telophase
The chromosomes begin to stretch outwards and lose their rodlike appearance. A new nuclear envelope eventually forms around each region of chromosomes.

Cytokinesis
The cell membrane is then pinched down in the middle. As a result, the cell splits into two. Each daughter cell ends up with the identical set of chromosomes as well as half of the organelles.
Specialized Cells

Red Blood Cells
Carries oxygen
Found in blood
Large surface area for oxygen to pass through
Consists of hemoglobin, which joins with oxygen
Consists of no nucleus, which increases surface area

Egg/Ovum Cells
Its purpose is to fertilize
It is found in the ovaries
Egg cells are usually bulky and large
These cells consists of a yolk which contains food storage for the new cell being formed
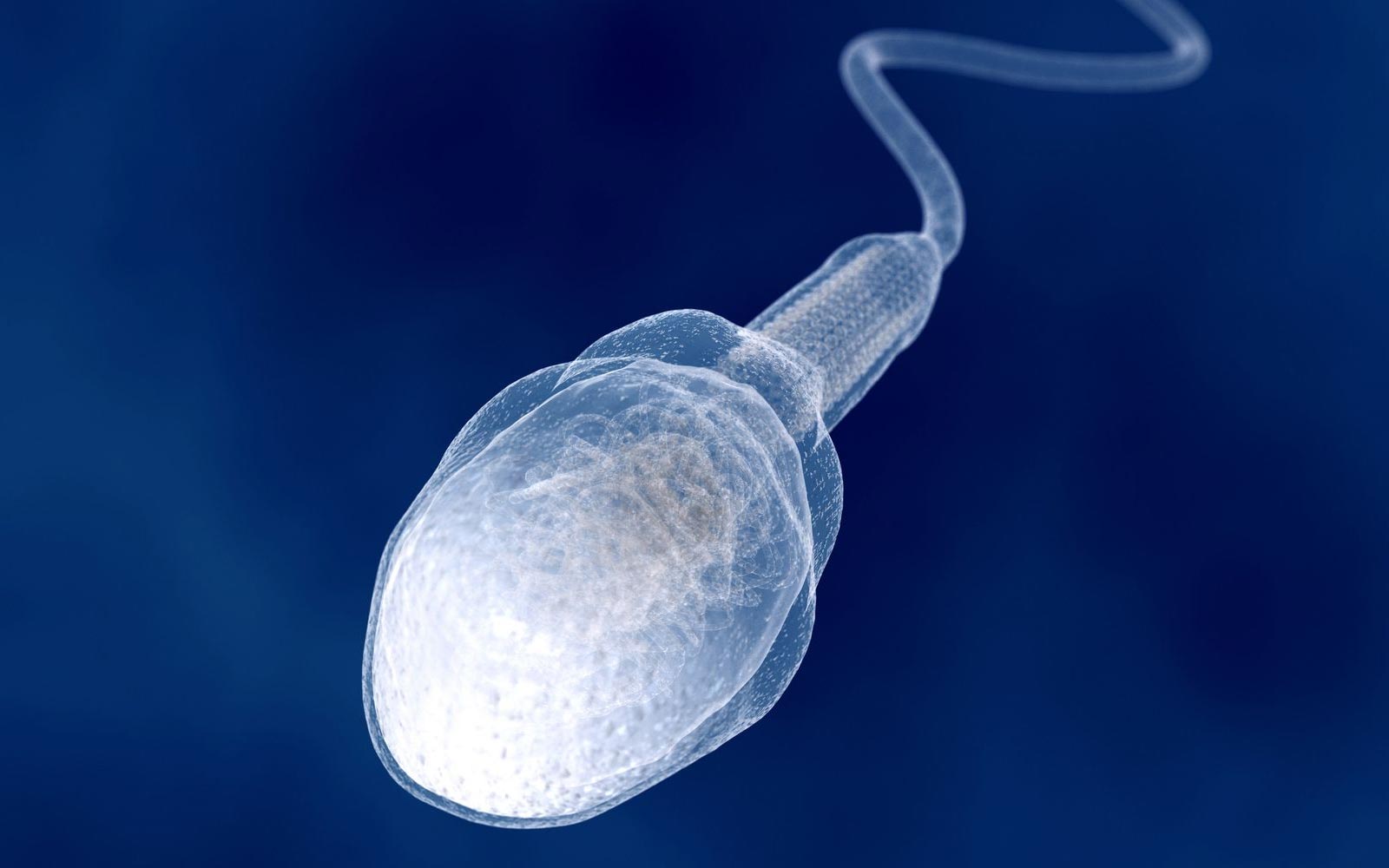
Sperm Cells
Its main purpose is to fertilize eggs
A single sperm is relatively small in size and has a long tail which provides movement, so it can swim and find the egg cell
It's found in the testes
The head consists of enzymes inside it which allows the cell to digest into an egg cell and join with it.
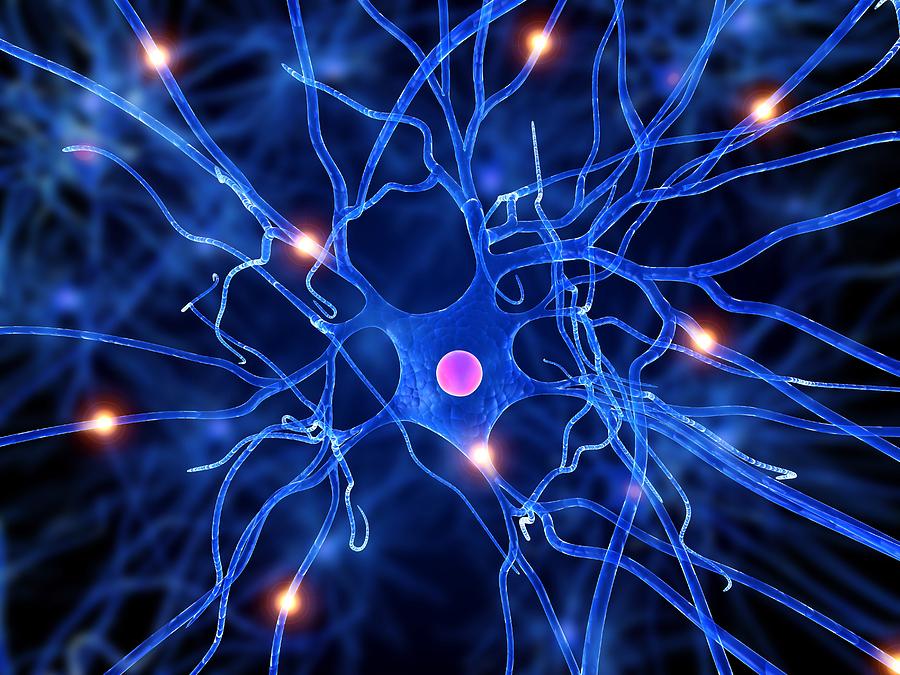
Nerve Cells
They have a long length
They have connections at each end
They have the ability to carry electric signals
Their main purpose is to carry nerve impules to different parts of the body

Root Hair Cells
Its purpose is to absorb
Always found in a plant root
Consists of a large surface which helps it absorb water and other nutrients
A thin cell wall makes it easier for minerals to pass through
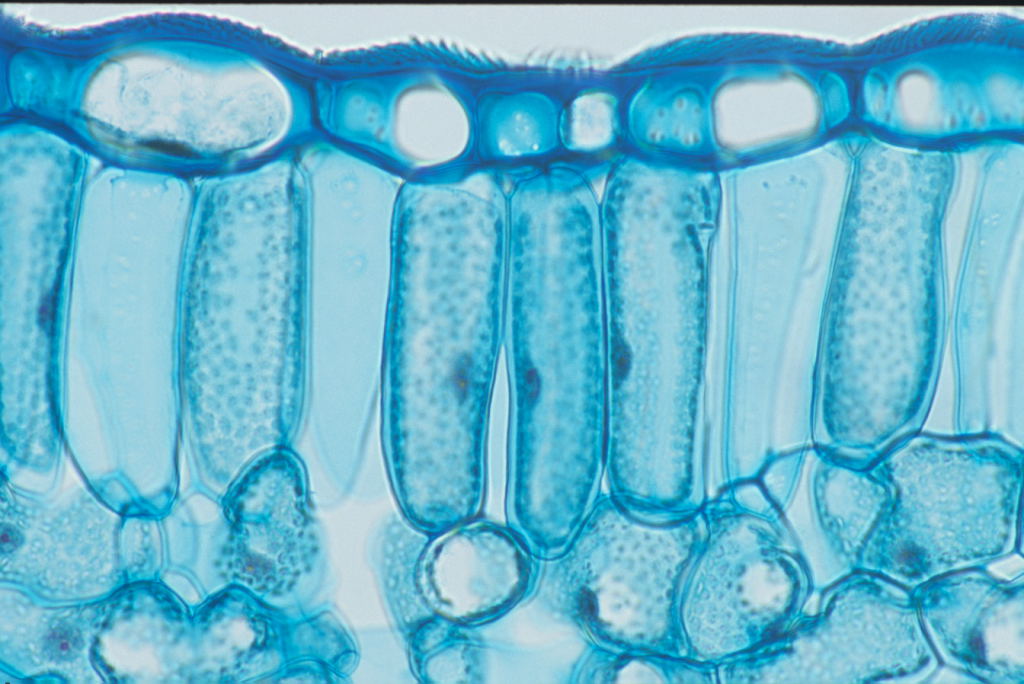
Palisade Cells
Its main purpose is to photosynthesize
It is found in the top of a leaf
It is relatively tall and has a large surface area to absorb nutrients
Consists of chloroplasts around it to help produce plant food
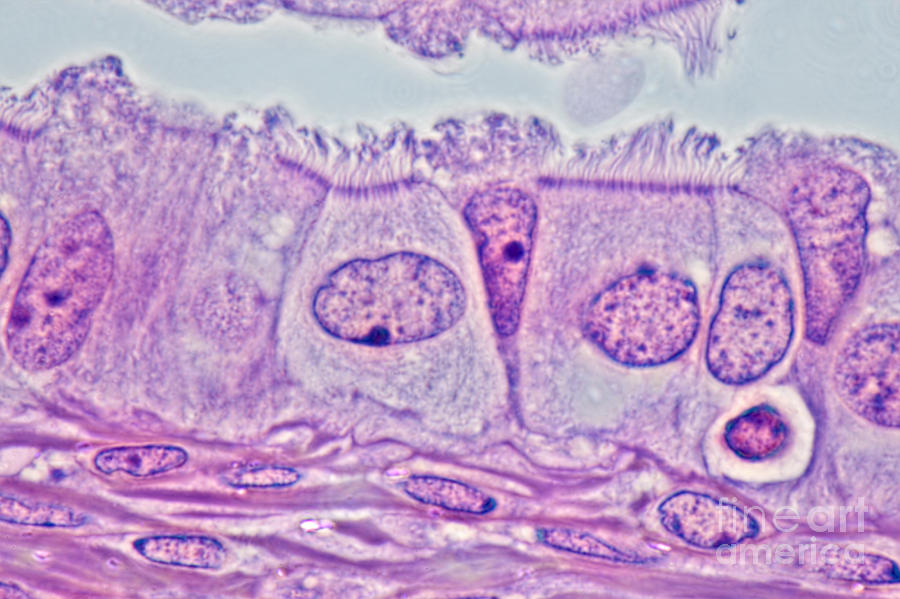
Clilated Cells
Their main purpose is to prevent lung damage
They line all the air passages in the lungs together
They consist of lots of cilia, which are tiny hairs
The cilia sweeps the mucus with trapped dust, and the bacteria backs up the throat
Human Body Systems
/GettyImages-97537745-59efa47d6f53ba0011c0070b.jpg)
Circulatory System
Major Parts

Blood

Heart

Arteries
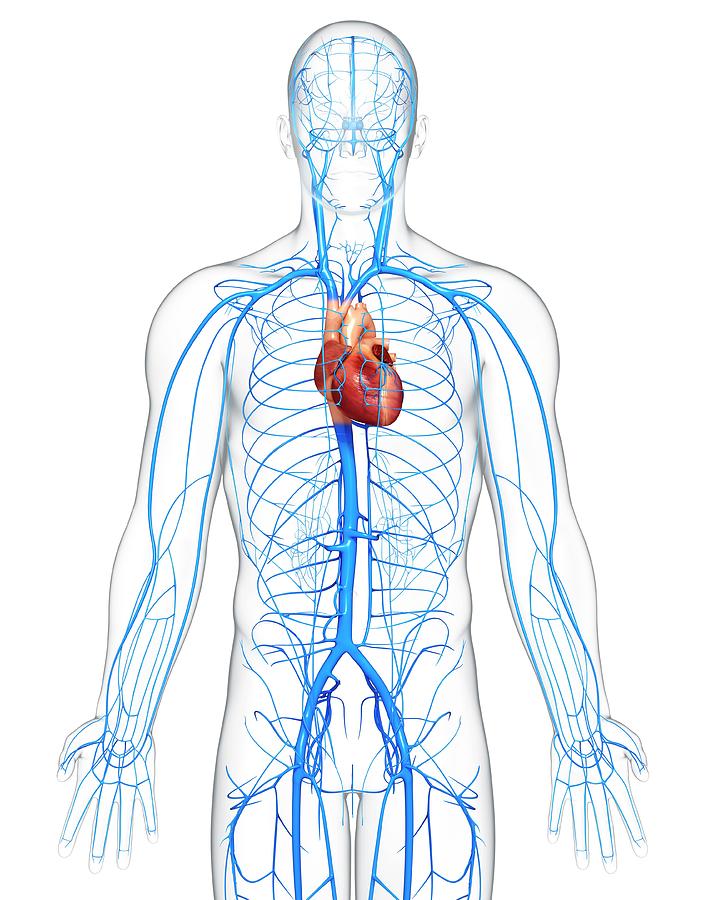
Veins
Responsibilities
Transport and distribute nutrients, oxygen and waste throughout the entire body

Respiratory System
Major Parts
Nose

Mouth
Lungs
Trachea
Responsibilities
Exchange carbon dioxide and oxygen
/human-digestive-system--artwork-136811327-599a0ae603f4020011c410a6.jpg)
Digestive System
Major Parts
Mouth

Esophagus

Stomach

Liver
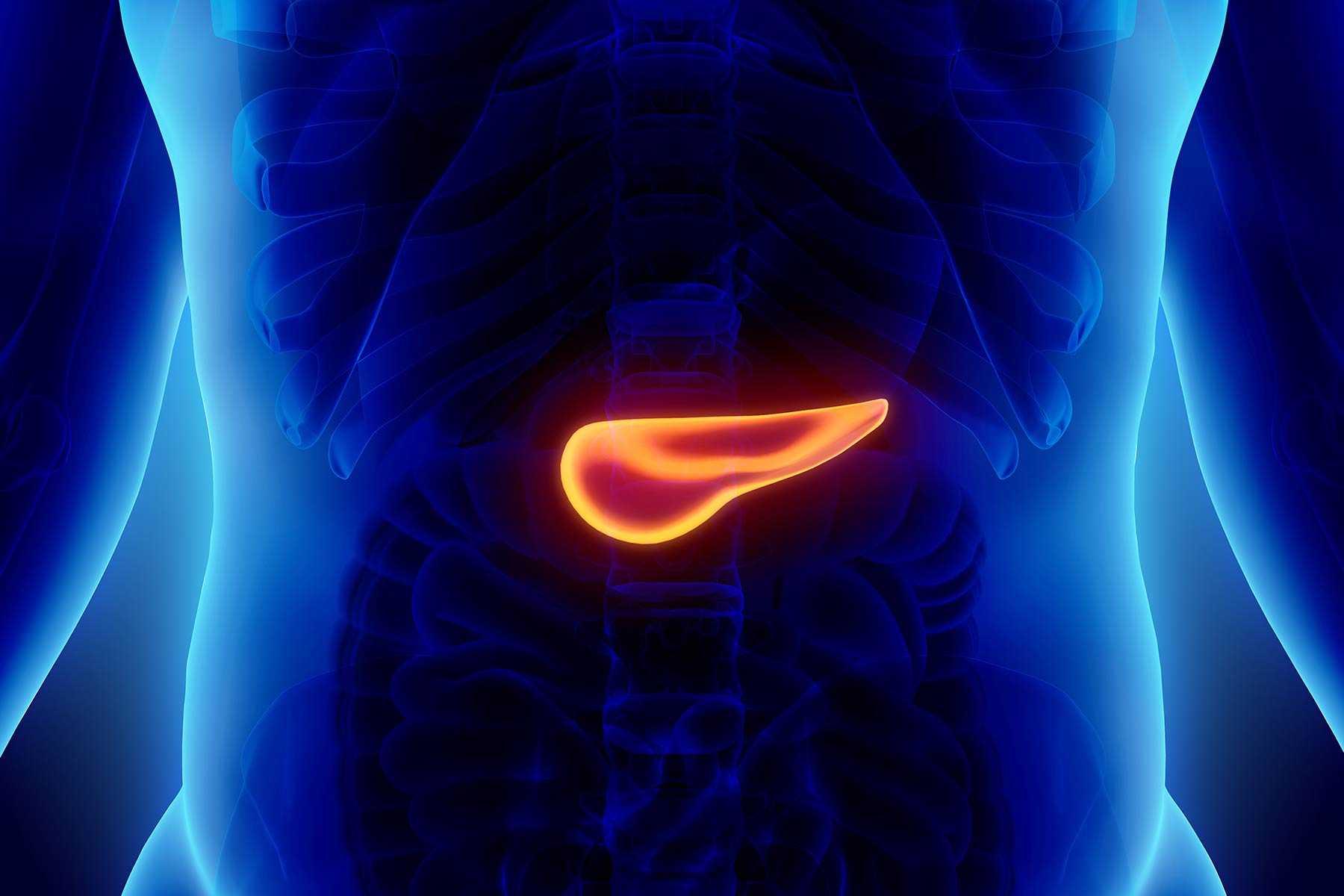
Pancreas
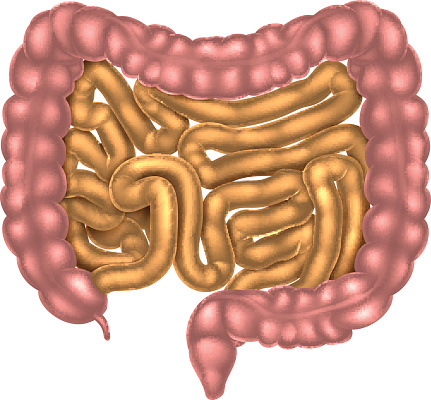
Intestines
Responsibilities
Break down food, absorb nutrients and eliminate waste
/male-nervous-system-artwork-168837900-578117135f9b5831b57baa42.jpg)
Nervous System
Major Parts
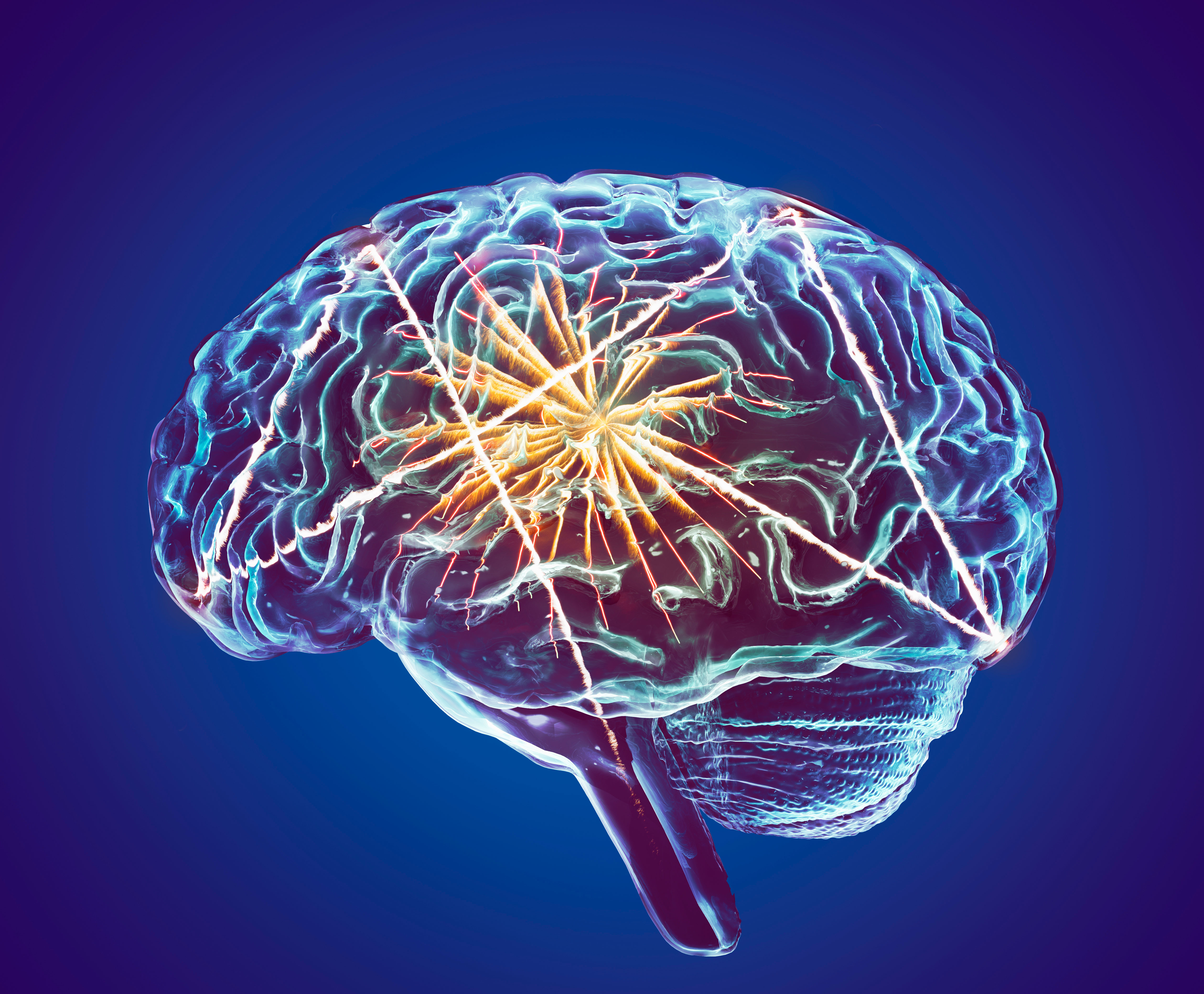
Brain
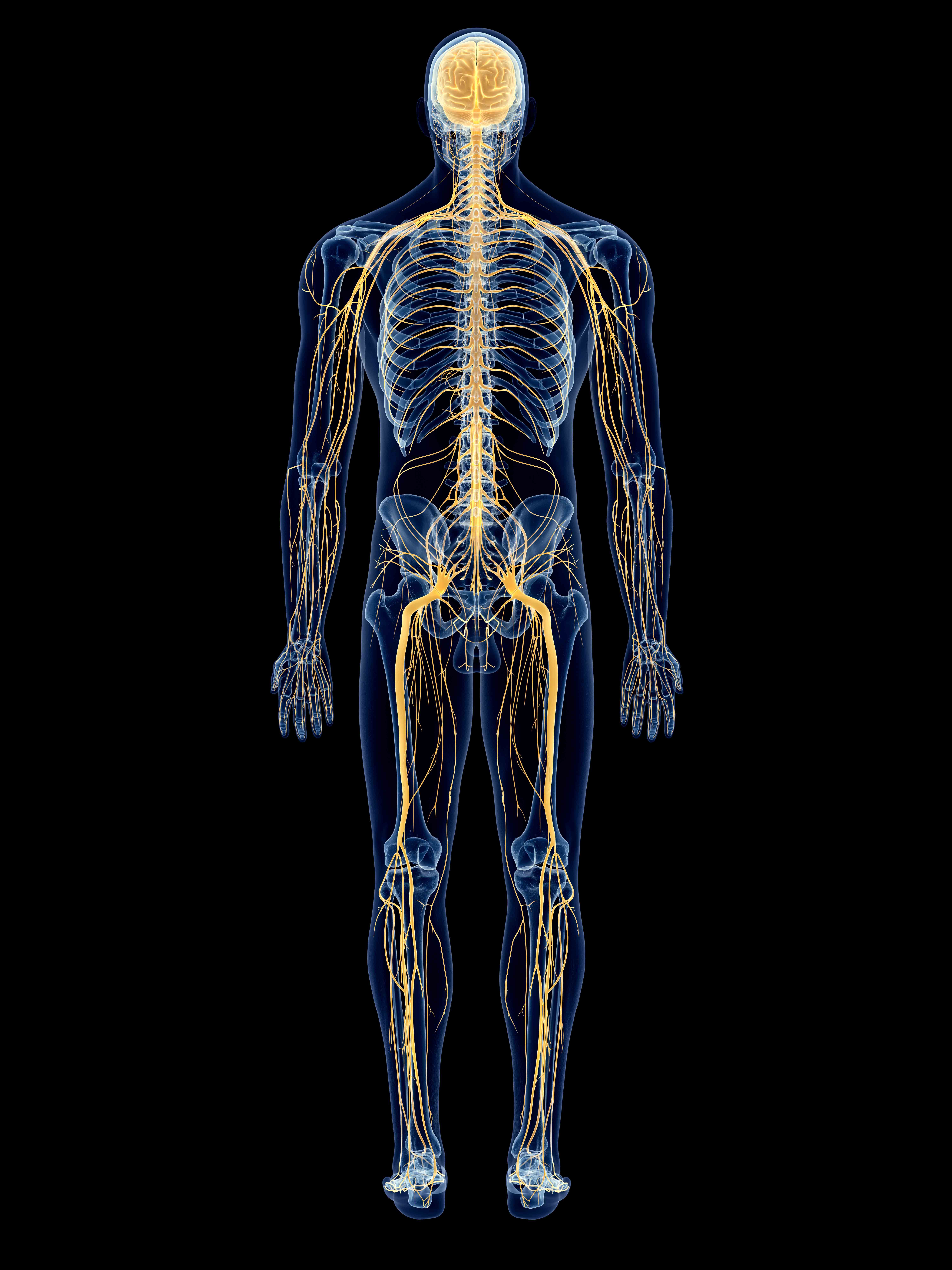
Nerves

Spinal cord
Responsibilities
Controls the senses as well as direct other systems

Urinary System
Major Parts

Bladder
Kidneys

Liver

Lungs
Rectum
Anus
/images/library/2747/cuiPO83hKxkwoMduGXV9A_Urethra__pars_membranacea_02.png)
Urethra
Responsibilities
Eliminating waste from blood
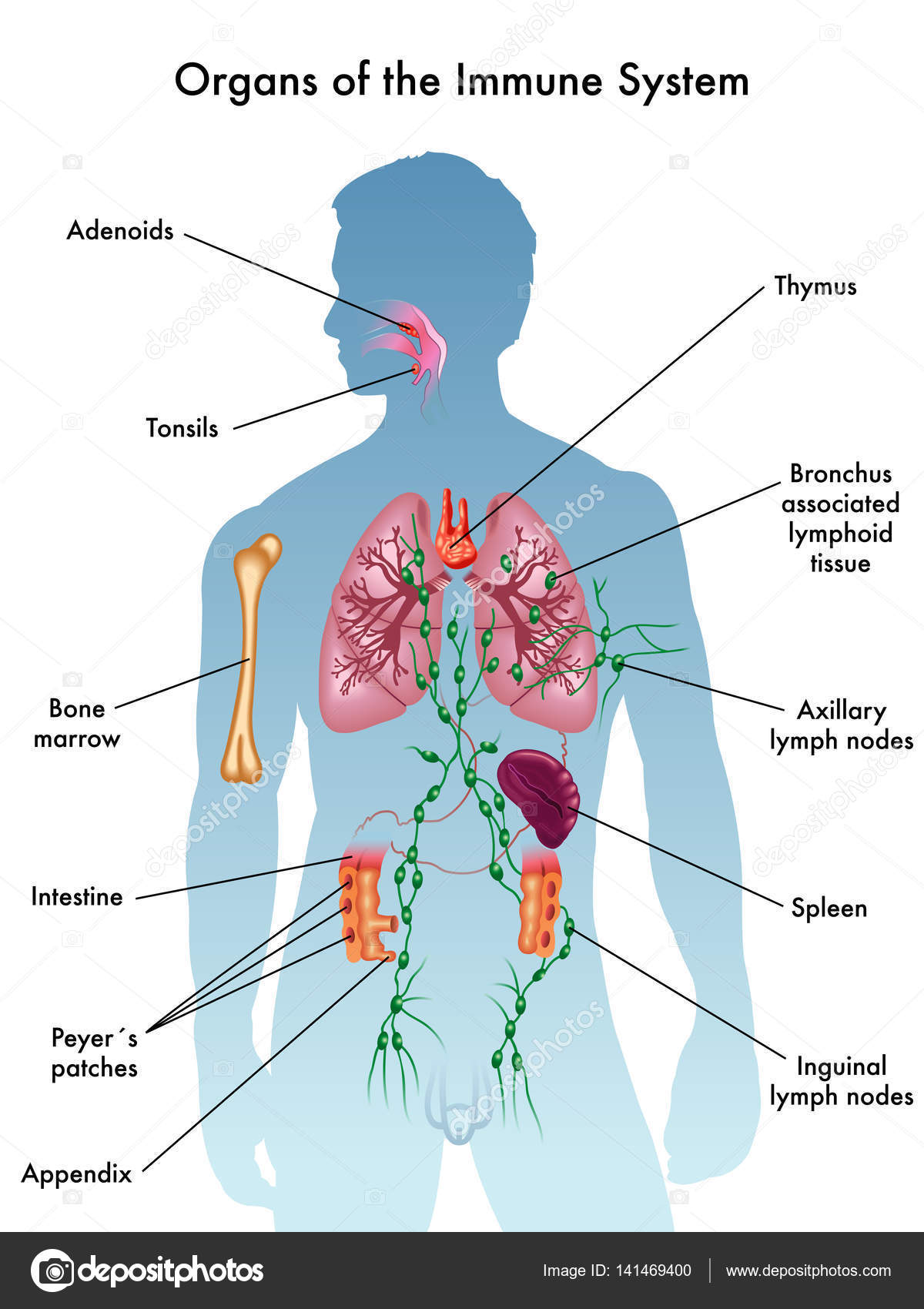
Immune System
Major Parts
White blood cells

T-cells

Antibodies
/160935452-56a5cfe45f9b58b7d0de8b7e.jpg)
Lymph nodes
Spleen
Bone marrow
Responsibilities
Protects the body from invaders, such as viruses

Skeletal System
Major Parts
Bone
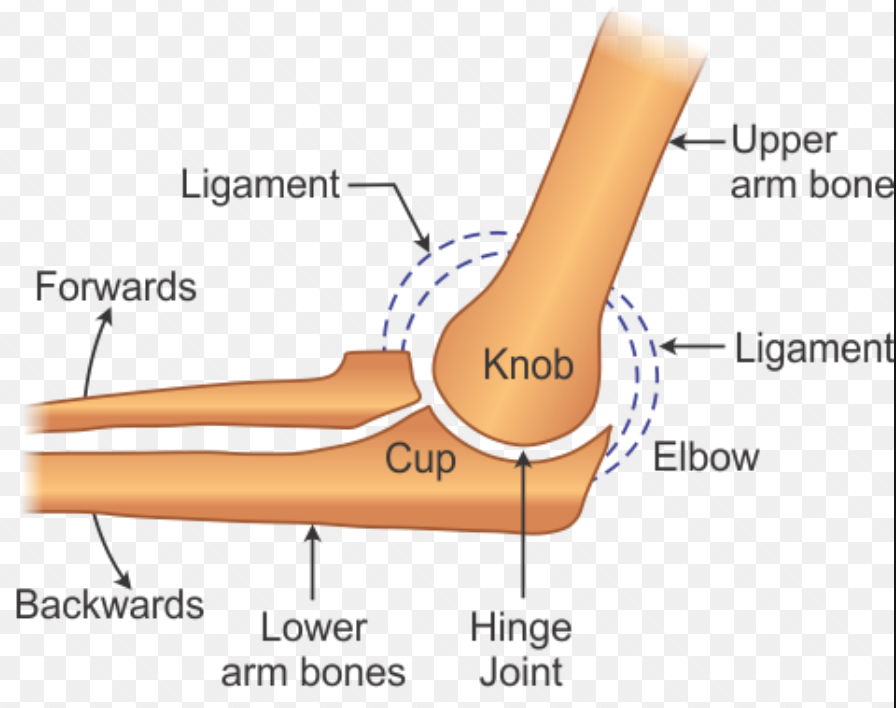
Joints

Marrow
Responsibilities
Protects and supports the organs, and producing red and white blood cells.

Microscopes
Types of Microscopes

Light Microscopes
These microscopes are mostly used in schools, and uses compound lenses to magnify objects. The lenses refract light to make the object beneath them appear a lot closer. Their common magnifications are 40x, 100x and 400x.

Stereoscopes
Much like binoculars, this microscope uses two-eye lenses to view larger specimens. Their lenses usually magnify 10x to 20x, but they can also be usable for larger specimens. They can even create 3D specimen views.

Scanning Electron Microscope (SEM)
This special microscope allows scientists to view a world too microscopic to be seen through a light microscope. SEMs do not use light waves; instead, they use negatively charged electrons to magnify at up to two million times. SEMs can also view specimens in 3D, but cannot view living specimens, as this might kill them.

Transmittion Electron Microscope (TEM)
TEMs also use electrons, however, instead of scanning the surface, electrons are passed through thin specimens.
Parts of a Microscope
Eyepiece
Body Tube
Revolving Nosepiece
Arm
Objective Lens
Stage
Stage Clips
Coarse Focus
Diaphragm
Fine Focus
Light
Base
Cell Organelles
Two Cell Types
Prokaryotes
No Nucleus
No Membrane Bound Organelles
Eukaryotes
Consists of a Nucleus
Consists of Membrane Bound Organelles
Two Main Types of Eukaryotic Cells
Animal Cell
Plant Cell

Cell Membrane
Surrounds the cell and determines what comes in and goes out
Absorbs nutrients in and lets waste out
Also known as Plasma Membrane
Found in plant cells, animal cells and prokaryotic cells
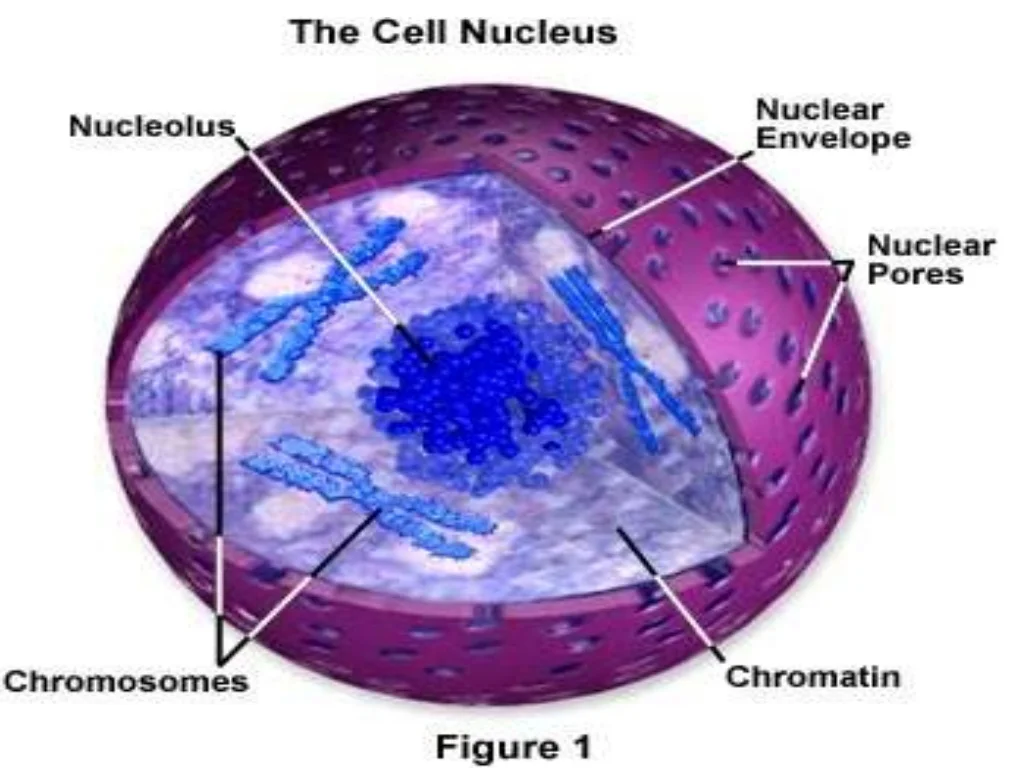
Nucleus
Controls the center of the cell
Stores and keep DNA
Surrounded by the nuclear membrane
Also consists of the nucleolus inside, which produces ribosomes
Found in plant and animal cells
Ribosomes
The smallest organelles
It is not surrounded by a membrane
Makes the proteins based on DNA instructions
Two types
Bound ribosomes are attached to rough ER
Free ribosomes float freely in cytosol
Found in plant, animal, and prokaryotic cells
/endoplasmic_reticulum-56cb365f3df78cfb379b574e.jpg)
Endoplasmic Recticulum
Transport the system for materials in the cell
Two types
Rough ER is covered with ribosomes; site of protein synthesis
Smooth ER has no ribosomes; it produces horomones and lipids instead
Found in plant and animal cells
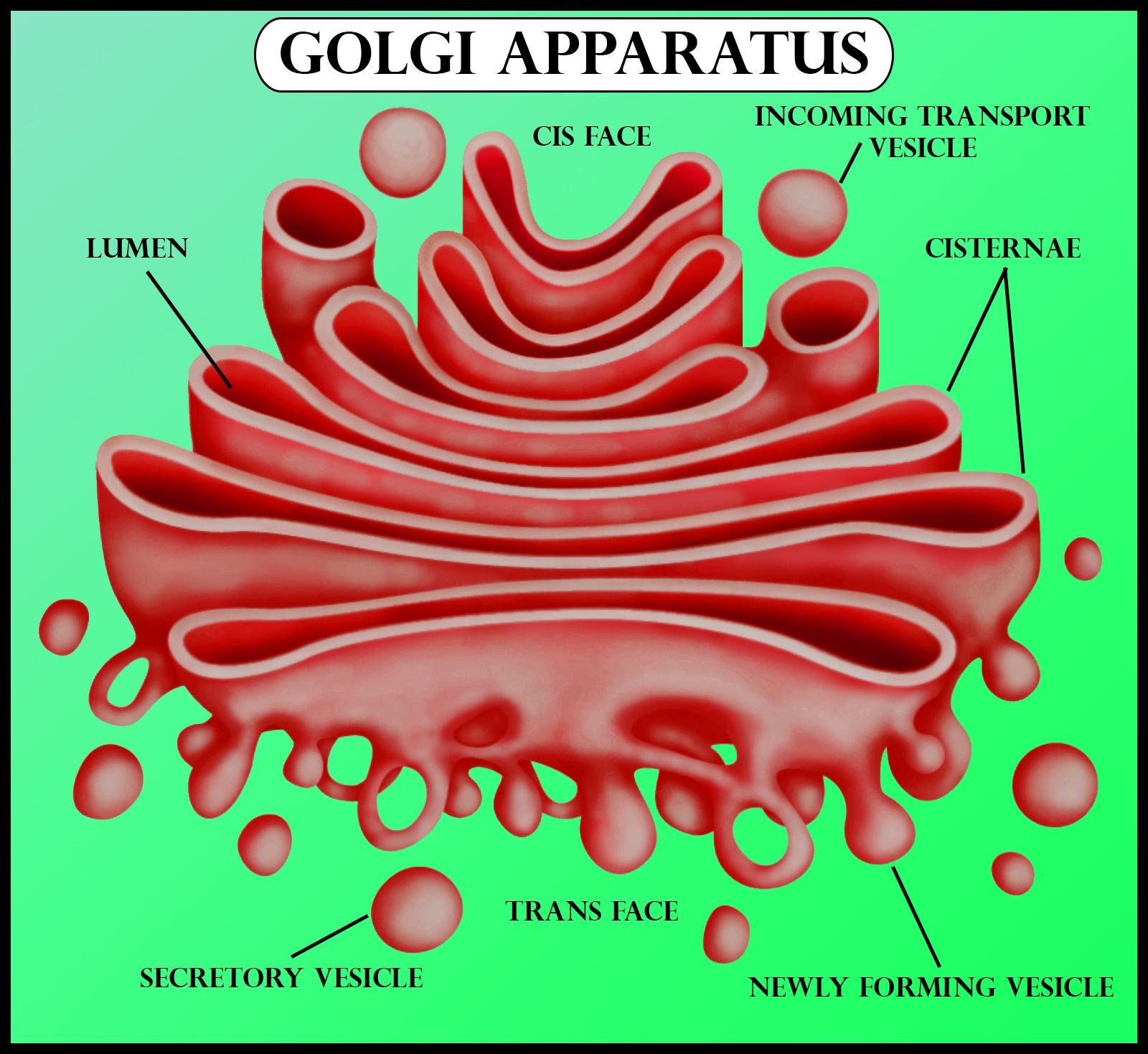
Golgi Apparatus
Functions as the delivery packaging system of the cell
Collects, modifies and packages cell molecules
Distributes and transports molecules through the vescicles
Found in plant and animal cells

Lysosomes
Functions as the trash disposer of the cell
Consists of digestive enzymes which breaks down waste
Found in plant and animal cells
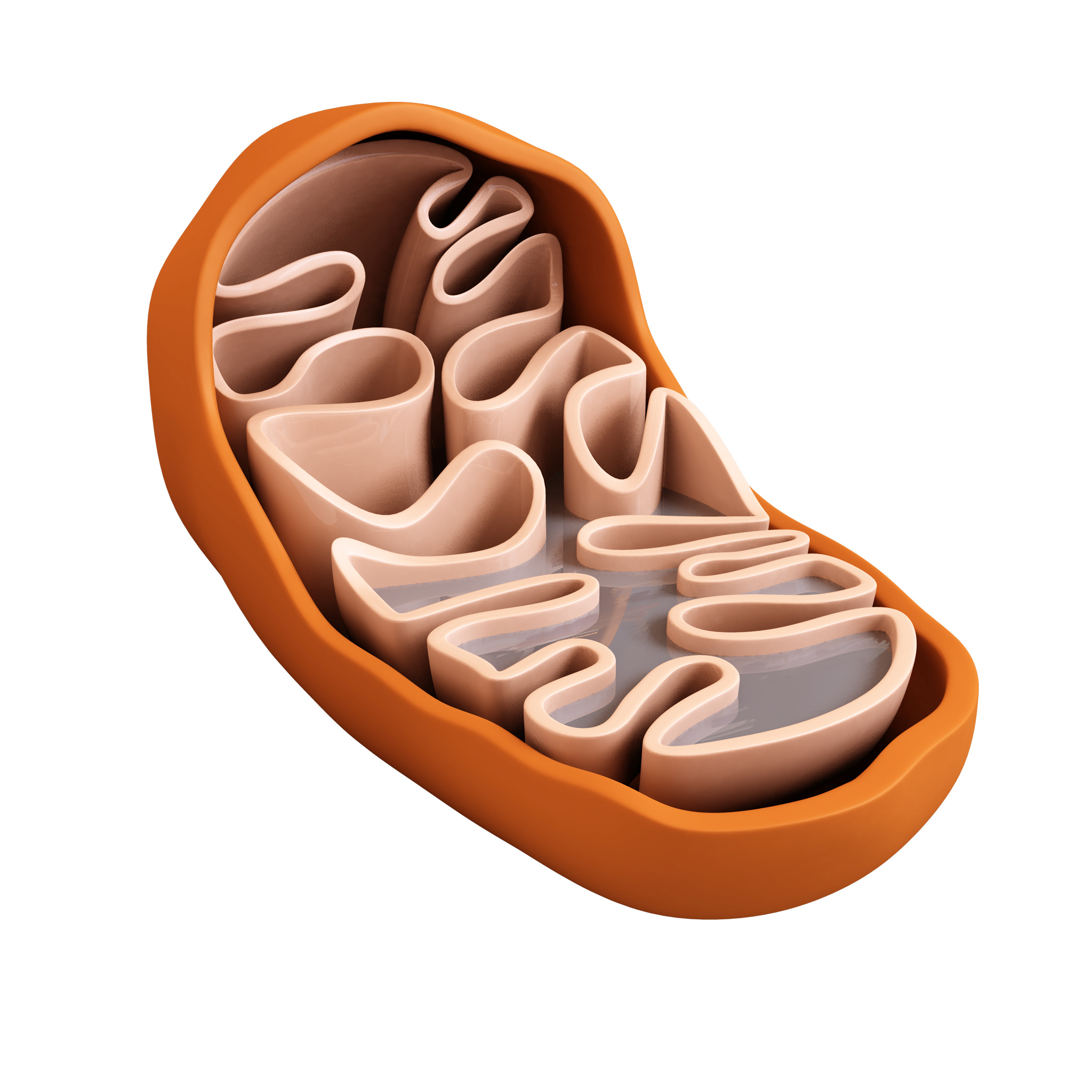
Mitochondria
Functions as the powerhouse of the cell
Site of celluar respiration
Changes energy which are stored in food to energy the cell needs; ATP
Found in plant and animal cells

Chloroplast
Found only in plant cells and algae
Consists of a green pigmnt; chlorophyll
Converts sunlight into food such as glucose

Cell Wall
Functions as a protective barrier for the cell
Found only in plant and bacteria cells
Located outside of the cell membrane
Made of celluose

Vacuoles
Large, centred vacuoles are usually in plant cells
Many smaller vacuoles are part of animal cells
Functions as the storage room for water, food, enzymes, waste, etc
Supports the cell shape in plants

Cancer
What is cancer?
Cancer can form and develop anywhere in the body, and it can also happen at any age.
Unlike other infectious diseases such as flu or the coronavirus, cancer is not contagious.
There are over 100 different types of cancer.
Statistics
1 in 4 deaths are caused by cancer
According to incident rates in 2009, it is estimated that 40% of Canadian women and 45% of men will develop cancer during their lifetimes.
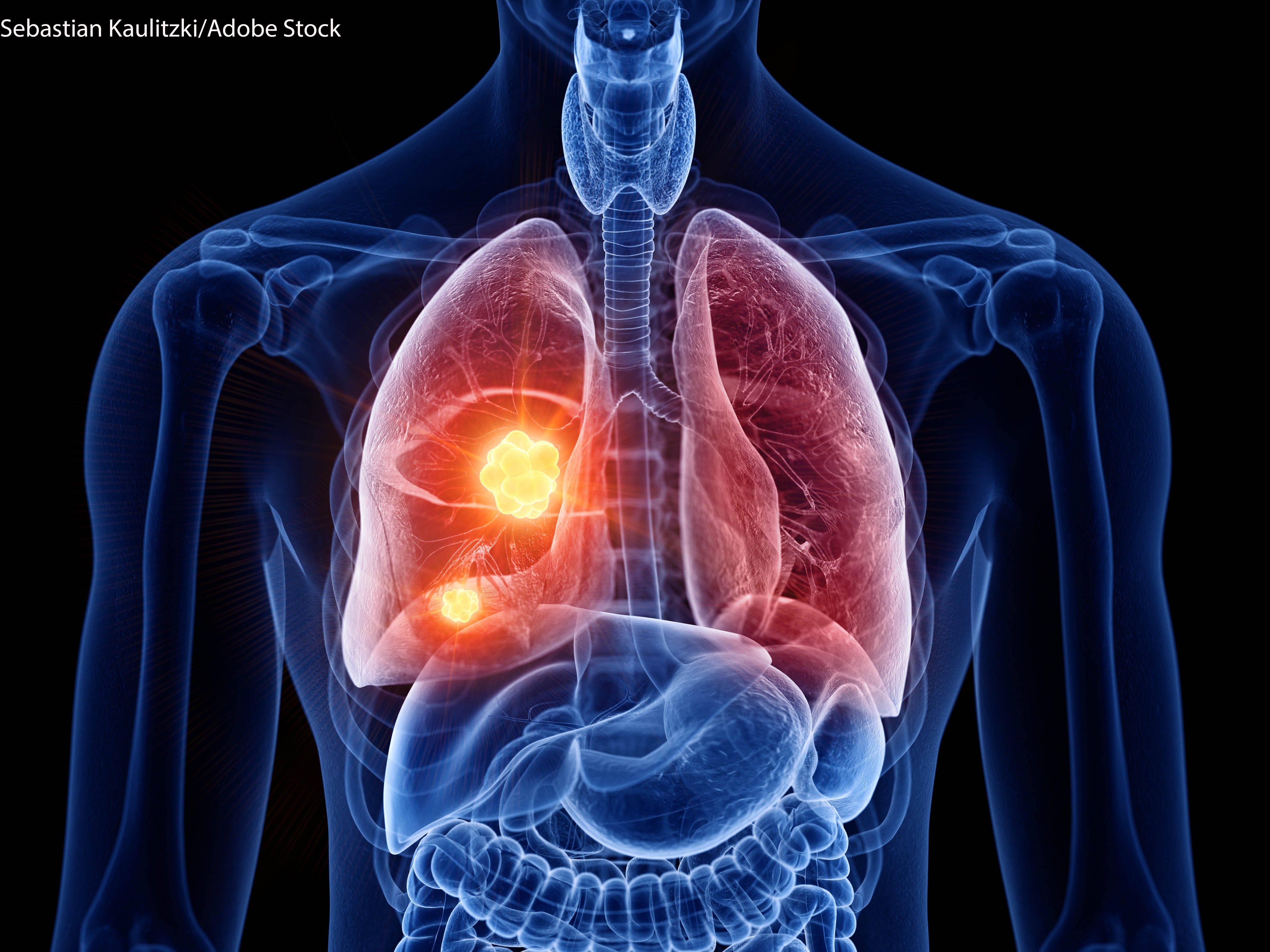
Lung Cancer remains as the leading cause of death for both men and women

Prostate cancer is the most common in men

Breast cancer is the most common in females
Two Tumor Types
Benign (non-cancerous) - does not spread, although it can sometimes be malignant
Malignant (cancerous) - has the ability to spread to other parts of the body
Preventing Cancer
Early detection of cancer is important. Cervical, breast and colorectal cancer screening as well as a self-exam for testicular cancer.

Make healthy choices - avoid tobacco, limit alcohol usage, protect your skin from UV, eat a healthy diet, maintain a healthy and appropriate weight and be more active

Get vaccines to reduce your chances of developing cancers later in life.
Safety and Lab Equipment
WHMIS (Workplace Hazardous Materials Information System)
WHMIS Symbols

Compressed Gas
Contents are under pressure
Explosion danger
Do not puncture, drop or place it near a heat source

Flammable & Combustible
Fire hazard
Flammable can cause fire at lower temperatures than combustible materials
Store in a cool area

Oxidizing Material
Fire/explosion risk
Keep away from open flames as well as combustable materials

Poisionous & Infectious: Acute
Can cause death if exposed to small amounts
May burn eyes or skin

Health Hazard
Poisonous over long-term exposure
May cause cancer, birth defects or sterility

Biohazardous
May cause serious diseases resulting in illness or death
Avoid contamination

Corrosive Materials
Can burn metals, skin or eyes
Avoid getting in contact with or inhaling

Explosive
Extremely reactive
Explosive hazard

Health/ozone layer hazard
Less critical health hazards
May cause extreme ozone damage
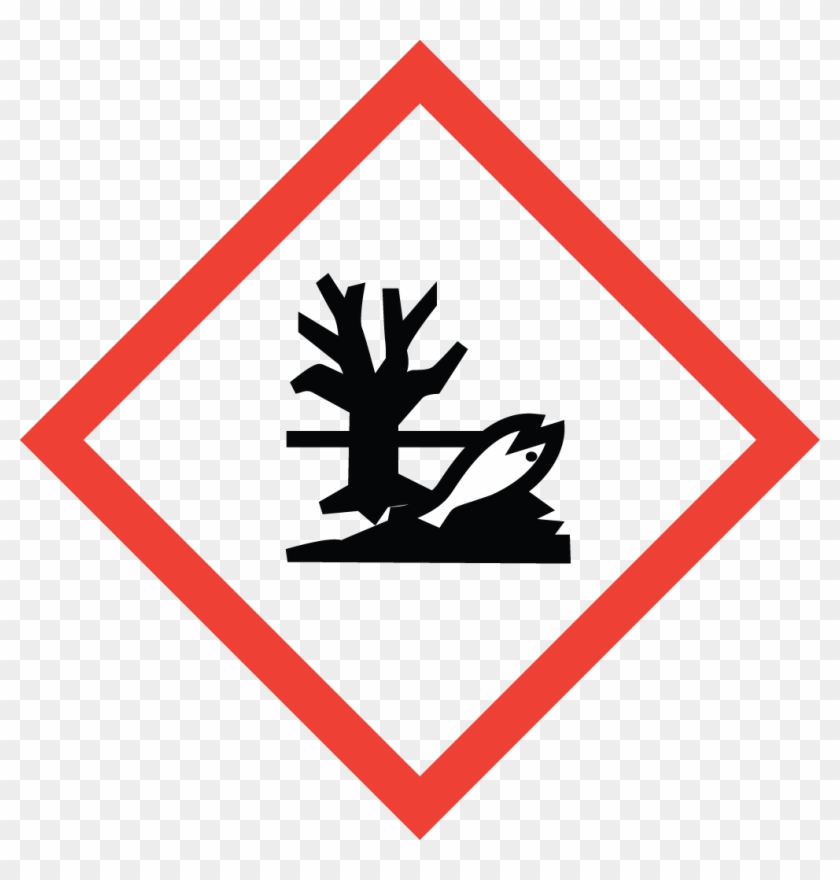
Environment
May cause damage to the aquatic environment
HHPS Symbols (Hazardous Household Product Symbols)
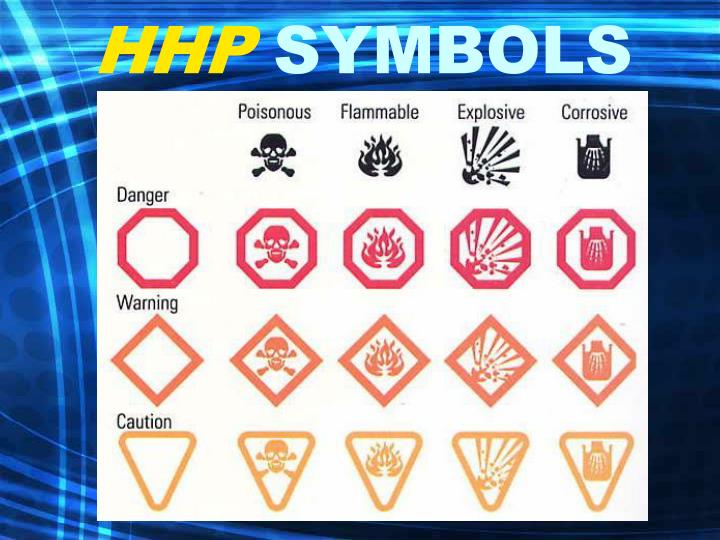
Lab Equipment
Glassware
Volumetric Glassware

Graduated Cylinder
A tall, narrow glass container with a volume scale
Used to measure liquids' volume ranging from 10 - 100 mL
Used for accurate measurement
Non-Volumetric Glassware

Beaker
A glass that consists of a wide mouth and a lip for pouring. Their sizes range between 50 and 1000 mL. Their main usage is for holding, mixing and heating liquids. They can also be used to transport liquids to another container. They are marked "graduated" on the side to indicate the approximate volume of their contents. Beakers are not appropriate for measurement accuracy.

Erlenmeyer Flask
These flasks are useful for mixing liquids by swirling them using a hand holding the neck. Like beakers, they are also marked "graduated" on the side to indicate the approximate volume of their contents. Erlenmeyer flasks are not appropriate for measurement accuracy.

Test Tubes
A lipped tube mainly used for heating, mixing or holding small amounts of solid or liquid chemicals
Other Equipment

Test Tube Rack
Used to hold 6 or 12 test tubes

Funnel
Used to pour liquids into containers which have small openings
Pipette
Used to transport a small quantity of a liquid

Tongs
Used for lifting containers that contains high temperature chemicals

Ring Stand & Clamp
A metal stand used containing a long, upright rod attached to a rectangular base. Used for supporting laboratory equipment

Thermometer Clamp
Used to hold thermometers on ring stands
Forceps
Used for pulling something, or for picking up objects which are too small to be handled

Triple Beam & Digital Balances
Triple beams are used to measure the masses; the reading error is 0.5 g. The mass ranges for balances are between 1g to 610g. Digital balances are used for measuring masses more accurately. The mass ranges for balances are between 0.5g to 600g.

Bunsen Burner
A common piece of laboratory equipment that produces a single flame. Used to heat substances for 100 degrees Celcius and higher
Goggles
Protective eyewear surrounding the eyes. Used to protect them from impact, dust, splashes, heat hazards, etc.

Test Tube Brush
Used for cleaning test tubes. They are located in beakers which are near to each sink.

Thermometer
A measurement tool used for determining the temperature, and also measures in Celsius

Petri Dish
A small, shallow dish made of glass or plastic with a loose cover. Used to hold a culture medium in which bacteria, fungi, germs, etc can be grown

Mortar & Pestle
Used to grind solid material into smaller pieces

Scoopula
Used to transfer solid substances, looks similar to a spoon

Evaporating Dish
Used to evaporate liquids in a solution

Watch Glass
Used to hold small quantaties of liquids or to cover an evaporating dish to prevent splattering

Crucible & Cover
Used to heat specific substances to remove water and then dry them

Wire Gauze
Usually placed between the bunsen burner and another equipment piece
Chemistry
Classification of Matter
Properties of Matter
Color (e.g, red, blue...)
Texture (e.g, soft, smooth...)
State of Matter (solid, liquid or gas)
Hardness (hard or soft)
Strength (weak or strong)
Temperature (hot or cold)
Odor (does it emit a smell)
Density (how dense is it)
Viscosity (depends on the speed of the flowing liquid)
Flammability (how easily does it catch on fire)
Three Kinds of Matter
Elements
Substances that cannot be broken down or made from any other substances. Always mentioned in the periodic table (e.g, oxygen, sulfur, lead, gold)
Compounds
A substance made from two or more elements which are chemically combined together
Mixtures
A substance made of two or more other substances which are not chemically combined
Homogeneous
These mixtures are not easy to tell the differences between the substances in them, and they are really difficult to seperate. They are also referred to as solutions.
Hetrogeneous
These mixtures are easy to tell the differences between the substances in them, and they are easy to seperate.
Solutions
Solute - a solid substance which is dissolvable in liquid
Solvent - the liquid that will dissolve that solid substance
Solution - a mixture which has the solute already dissolved in the solvent
Atoms and Ions
Parts of an Atom
Nucleus - the center of the atom which consists of protons and neutrons
Orbitals/Shells - areas around the nucleus which contains electrons that circulate around it
Subatomic Particles
Protons - has a positive charge, found in the nucleus, and weighing a specific amount
Neutrons - has a neutral charge, also found in the nucleus, and weighing a specific amount
Electrons - has a negative charge, found circulating the nucleus through shells, and does not weigh a specific amount
Valence electrons - the outermost electrons in the outermost/largest shell
Periodic Table
Atomic symbol - abbreviations which are used for elements in the periodic table (e.g, zinc = Zn)
Atomic number - represents the number of protons, and also the number of electrons in a neutral atom. Since each atoms have different amounts of protons, the atomic number is used to order the elements. (e.g, chromium = 24)
Atomic mass - indicates the total number of protons and neutrons (e.g, flourine; 10 neutrons + 9 protons = 19 (atomic number))
Ions
Anions = negative ion
Cations = positive ion
Charged atoms or molecules
Compounds
Ionic Compounds
Ionic compunds will result when nonmetals take electrons from metals
Metals lose electrons to form positive ions as a result
Non-metals gain electrons to form into negative ions as a result
Polyatomic Compounds
Polyatomics are groups of atoms that are stable and have a net charge
Molecular Compounds
A type of compound formed when two or more atoms of different elements share electrons
Usually formed between 2 or more nonmetals; also referred to as covalent compounds
Acids & Bases
Acids
Compounds which dissolves in water to produce hydrogen ions in solution
Properties
Tastes soury
Reacts with some metals to produce hydrogen
pH of less than 7
Indicators
Blue litmus turns red
Pink pheno turns into no color
Neutral blue bromothymol turns into yellow
Orange methyl turns red
Bases
Compounds which dissolves in water to porduce hydroxide ions in solution
Properties
Tastes bittery
Feels slippery and soapy
pH of more than 7
Indicators
Red litmus turns blue
The colorless pheno turns pink
Blue bromothymol turns blue
Orange methyl turns in a yellow-orange color
Cabbage juice turns in a blue-green color
pH scale
The pH scale is a measurement scale used to determine how acidic or basic a solution is
The pH scale also measures how many hydrogen or hydroxide ions are there in the solution
An acidic solution has a low pH (typically below 7)
A basic solution has a high pH (typically above 7)
A neutral solution has a pH of around 7 (e.g, pure water)
Indicators
Indicators are chemicals that turn into different colors when added to an acid or base
Common Indicators

Red litmus

Blue litmus

pH paper

Cabbage juice

Phenolphthalein

Bromothymol blue
Scientific Method
Steps
Problem/Question/Purpose
When we develop a specific question or problem, we can solve it using experimentation to see the outcome.
Observation/Research
We make our observations and research our topic based on the experiment.
Hypothesis
Predict an answer to your problem or question. A hypothesis is an educated guess about the independent and dependent variables.
Independent Variable
A factor that can be controlled or changed during an experiment. Valid experiments have only one independent variable.
Dependent Variable
A factor that might be changed as a result of the independent variable changes. The dependent variable, however, must be measured.
Procedure
Develop and follow the desired procedure.
Materials
Include a detailed materials list.
Collect & Analyze Results
Change the procedure when needed. Confirm the outcome by retesting. Record your outcome using graphs, tables, photos, etc.
Conclusion
Using from what you experienced from your outcome, include your statement on whether it accepts the hypothesis or rejects it. Use recommendations to further improve your experiment and to study more about it.