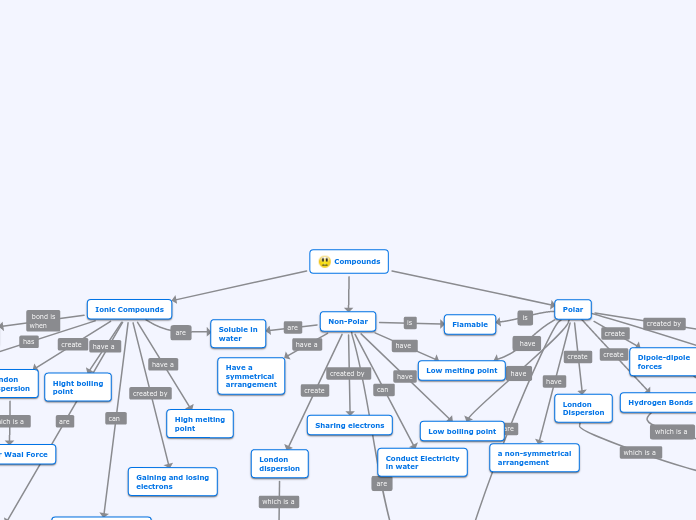
Compounds
Polar
A metal and a
non-metal reacting
a non-symmetrical
arrangement
London
Dispersion
Van Der Waal Forces
Dipole-dipole
forces
Hydrogen Bonds
Soluble in water
Soft and squishy
Ionic Compounds
Negative and positive
ions attract electrons
London
dispersion
Van Der Waal Force
High melting
point
Hight boiling
point
Gaining and losing
electrons
Low flamibility
Conduct electricity in
water
Hard and brittle
Non-Polar
Low melting point
Low boiling point
Sharing electrons
Soluble in
water
Have a
symmetrical
arrangement
London
dispersion
Van Der Waal
Force
Conduct Electricity
in water
Flamable