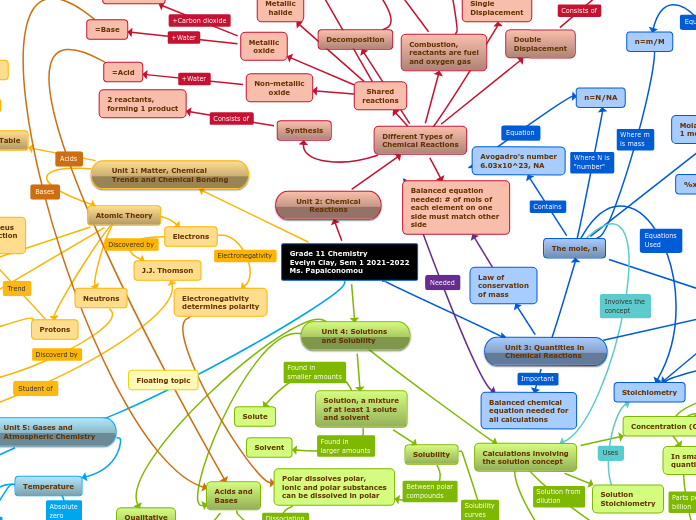
Grade 11 Chemistry
Evelyn Clay, Sem 1 2021-2022
Ms. Papaiconomou
Unit 3: Quantities in
Chemical Reactions
The mole, n
Avogadro's number
6.03x10^23, NA
Molar mass, mass of
1 mol of a substance, M
n=m/M
n=N/NA
Calculations involving
the mole concept
Percentage
composition
%xtotal=(mx/mtotal)*100%
Empirical and
molecular formulas
Finding simplest whole #
ration of atoms/ions
Finding actual # of
elements in a compound
Limiting and
excess reagents
Reactant that
is used up in
reaction
The smaller
mole product #
Reactant that is
not used up first
nproduct=nhave*(#of mols reactant)
(# of mols product)
Percentage yield
%yield=(actual yield) * 100%
(theoretical yield)
Amount of product
that could be produced
Amount of product
actually produced
Stoichiometry
nwant=nhave*(#of mols want)
(# of mols have)
Law of
conservation
of mass
Balanced chemical
equation needed for
all calculations
Unit 2: Chemical
Reactions
Different Types of
Chemical Reactions
Synthesis
2 reactants,
forming 1 product
Decomposition
One product,
breaking down
into 2 reactants
Combustion,
reactants are fuel
and oxygen gas
Incomplete
Combustion
Products are CO2 gas,
water vapour and energy
Exothermic reaction
produces thermal energy
Soot (solid carbon)
and carbon monoxide
Flame is orange,
black soot produced
Complete
Combustion
Flame is blue or
invisible
Single
Displacement
Metal
Lone metal replaces
metal in compound
Only occurs if lone metal
is higher on activity series
than metal in compound
Hydrogen gas and metal
hydroxide OR ionic compound
produced
Halogen
Lone halogen replaces
halogen in compound
Double
Displacement
2 reactants,
forming 2 products
Each reactant has
2 elements, molecules,
polyatomic ions etc.
Balanced equation
needed: # of mols of
each element on one
side must match other
side
Unit 1: Matter, Chemical
Trends and Chemical Bonding
Periodic Table
95 Metals
Group 1:
Alkali Metals
Group 2: Alkaline
Earth Metals
Groups 3-12:
Transition Metals
Mostly solid at SATP,
conduct heat and
electricity well
A metal and non-metal
can form an ionic
compound
17 Non-metals
Group 18:
Noble Gases
Unreactive due to
full valence shell
Group 17:
Halogens
6 Metalloids
Staircase line
Shiny like metals,
brittle like non-metals
Dmitri Mendeleev
Horizontally,
by period
-Nuclear charge increases
-Shielding effect is constant
-Atomic radius decreases
-Ionization energy increases
-Electronegativity increases
-Electron affinity increses
Vertically,
by Group
-Nuclear charge increases
-Shielding effect increases
-Atomic radius increases
-Ionization energy decreases
-Electronegativity decreases
-Electron affinity decreases
Repulsion
of valence electrons
by inner electrons
More protons in nucleus
means greater attraction
of valence electrons
Mendeleev's periodic law:
Elements arranged by
increasing atomic mass
Modern periodic law:
Elements arranged by
increasing atomic number
Atomic Theory
Electrons
J.J. Thomson
Electronegativity
determines polarity
Protons
Ernst Rutherford
Alpha, Beta,
Gamma Rays
Neutrons
James
Chadwick
Nucleus
Energy
Levels
Neils
Bohr
Unit 5: Gases and
Atmospheric Chemistry
Temperature
Kelvin=Celsius+273
Celsius=Kelvin-273
0k or -273 Celsius
Pressure
1 atm=101.325kPa
760torr=760Hgmm
760Hgmm=1atm
Gas
Laws
Boyle's
Law
P1V1=P2V2
Charle's
Law
V1=V2
T1 T2
Amonton's
Law
P1=P2
T1 T2
Ideal
gas law
n=PV
RT
Ideal gas
constant:
8.31 kPaL/molK
Combined
gas law
P1V1=P2V2
T1 T2
Gas
Stoichiometry
n=v
Vmolar
Vmolar=22.4L/mol
Vmolar=24.8L/mol
Stoichiometry
(Unit 3)
Unit 4: Solutions
and Solubility
Solution, a mixture
of at least 1 solute
and solvent
Solute
Solvent
Solubility
Polar dissolves polar,
ionic and polar substances
can be dissolved in polar
Ionic substances with
+ and - parts get pulled
apart by polar substances
Temp vs g dissolved
per 100g of H2O
Unsaturated
solution
Saturated
solution
Supersaturated
solution
Acids and
Bases
Base
A substance that turns
red litmus paper blue
Dissociates in water
to form [OH-] ions
8-11
pH and [H+]
concentration
-log[H+]
10^-pH
pH of 7
Acid
Calculations involving
the solution concept
Concentration (C)
C=Amount of solute(n)
Volume of solution(L)
%=(Mass of solute (g)) x100%
(Volume of solution (mL))
%=(Mass of solute (g)) x100%
(Mass of solution (g))
%=(Volume of solute (ml)) x100%
(Volume of solution (mL))
In small
quantities
ppm=(Mass of solute (g)) x10^6
(Mass of solution (g))
ppb=(Mass of solute (g)) x10^9
(Mass of solution (g))
CiVi=CfVf
Requires molar concentration
equation and n=m/M equation
Solution
Stoichiometry
Qualitative
Analysis
Some ions turn
a different colour
in solution
Some ions turn
flame into a
different colour
Sequential
chemical
analysis
Used to find ion presence
if a precipitate forms or not
Representative
element: closely
follow periodic law
Metallic
oxide
=Base
=Carbonate
Metallic
halide
=Chlorate/bromate/iodate
Non-metallic
oxide
=Acid
Element
=Binary
compound
Shared
reactions
Can be dangerous, CO
prevents oxygen transport
in the body
A substance that turns
blue litmus paper red
Ionizes in water
to form [H+] ions
1-6