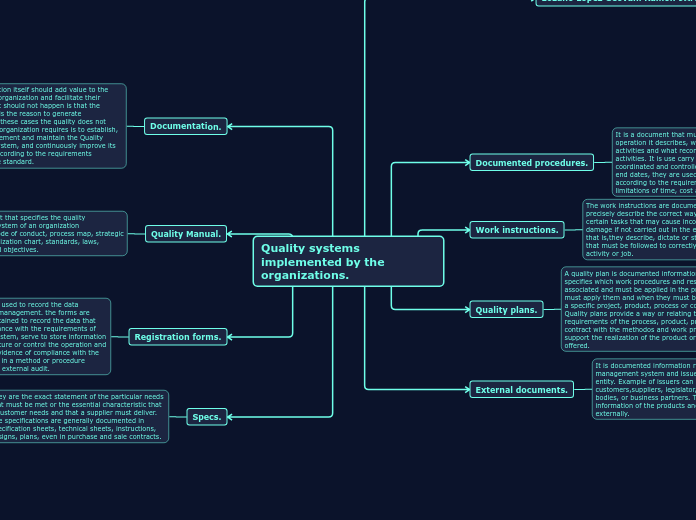
Quality systems implemented by the organizations.
Lozano López Geovani Ramón 9AM
Documented procedures.
It is a document that must accurately reflect the operation it describes, who is responsible for the activities and what records arise from those activities. It is use carry out a set of activities in a coordinated and controlled manner with start and end dates, they are used to archives an objective according to the requirements, it includes the limitations of time, cost and resource.
Work instructions.
The work instructions are documents that clearly and precisely describe the correct way to carry out certain tasks that may cause inconvenience or damage if not carried out in the established manner. that is,they describe, dictate or stipulate the steps that must be followed to correctly perform a specific activity or job.
Materials.
Protective equipment
Manuals.
Quality plans.
A quality plan is documented information that specifies which work procedures and resources are associated and must be applied in the process, who must apply them and when they must be applied to a specific project, product, process or contract. Quality plans provide a way or relating the specific requirements of the process, product, project or contract with the methodos and work practices that support the realization of the product or service offered.
Specification of the service.
Specific rules.
Description of the processes.
Documentation.
Responsibilities.
Organization chart.
External documents.
It is documented information relevant to the quality management system and issued by an external entity. Example of issuers can be: customers,suppliers, legislator, regulators, standards bodies, or business partners. They contain the information of the products and services contracted externally.
Service specifications.
Documents.
Laws.
Norms.
Documentation.
The documentation itself should add value to the activities of an organization and facilitate their execution. What should not happen is that the documentation is the reason to generate bureaucracy, in these cases the quality does not exist. what the organization requires is to establish, document, implement and maintain the Quality Management System, and continuously improve its effectiveness according to the requirements contained in the standard.
The Declaration of the Quality Policy and Quality Objectives.
The Quality Manual.
The procedures and records that the norm requires.
The documents that the organization establishes to ensure the effectiveness of the planning, operation and control of its processes.
The records that the standard requires.
Quality Manual.
It is a document that specifies the quality management system of an organization (Regulations,Code of conduct, process map, strategic planning, organization chart, standards, laws, instructions and objectives.
Organization for quality.
Quality function planning.
Control of the project or design.
Relationship with suppliers.
Process control.
final control and tests.
Special processes.
Registration forms.
They are documents used to record the data required for quality management. the forms are developed and maintained to record the data that demonstrate compliance with the requirements of the management system, serve to store information of an informative nature or control the operation and serve as objective evidence of compliance with the activities carried out in a method or procedure before an internal or external audit.
ID.
Storage.
Protection.
Recovery.
Retention.
Disposition of records.
Specs.
They are the exact statement of the particular needs that must be met or the essential characteristic that a customer needs and that a supplier must deliver. The specifications are generally documented in specification sheets, technical sheets, instructions, designs, plans, even in purchase and sale contracts.
Procedures.
Process specifications.
Product specifications.
Performance specifications.