por Wardina Syasya hace 4 años
290
4.2 Standards
por Wardina Syasya hace 4 años
290

Ver más

To name your story, you have to think about the overall message and what you want your audience to understand from the story. Also, make it relevant and easy to remember.
The ending of a story is essential. We all know that if the ending is weak, what happened before loses its importance. So make it unpredictable, but fair. A resolved ending answers all the questions and ties up any loose threads from the plot.
This is the closure section of the story.
See examples of possible outcomes below:
Try answering these questions in order for you to come up with a closure:
- Have all problems been solved?
- Is it clear what happens with all your characters in the story?
- Has the challenged transformed your main character?
- How do the characters feel in the end?
Try answering these questions to come up with a closure:
- Have all the problems been solved?
- Is there a clear picture of what happens with each character in the story?
- Has the challenge transformed your main character?
- How do the characters feel in the end?
This is the moment when the main character surpasses the last obstacle and finally faces their greatest challenge.
The climax usually follows one of these patterns:
Type in your answer.
The middle of the story is where you add layers of complications that will lead to the end. Reveal more about the character's journey. Did their personality go through changes? How did they overcome the challenges? And as you build up the story’s central conflict, make it more personal to that character. Also, from the middle act, you have to lead into the final act.
There wouldn't be any tension and excitement in your story if there weren't any obstacles in your character's way.
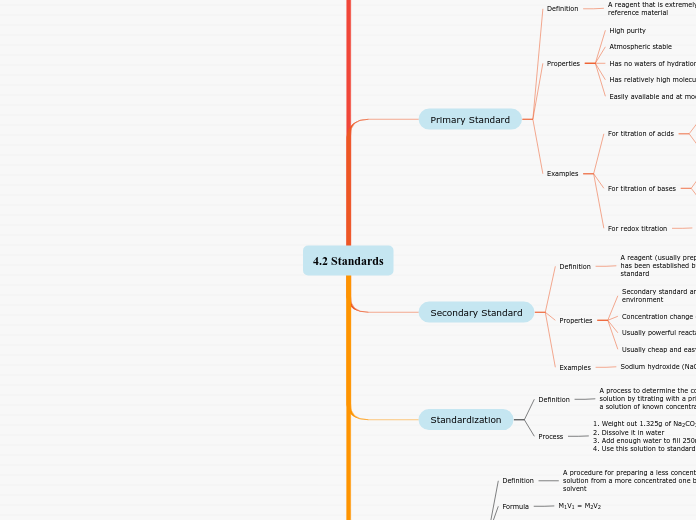
potassium dichromate : K2Cr2O7, mol wt= 294.19 g/mol
ii) potassium hydrogen iodate : KH(IO3)2, mol wt= 289.92 g/mol
i) potassium hydrogen phthalate (KHP): KHC8H4O4, mol wt= 204.23 g/mol
A story is nothing more than a character overcoming a series of difficulties to reach the desired goal. Obstacles usually create suspense and conflict. In overcoming obstacles, there is growth: weak becomes strong; hatred turns into love; sadness into happiness; wrong into right; lies into truth; or evil becomes good.
See a few examples below:
ii) tris-(hydroxymethyl)aminimethane, (CH2OH)3CNH2 mol wt= 121.12 g/mol
i) Sodium carbonate, Na2CO3, mol wt= 105.99 g/mol
Your character(s) need(s) motivation in order to solve the challenge(s).
Secondary characters also might have motivs beacuse of which they may cross path with main character or which might trigger them to help the main character.
Each story has a main character and that character usually needs to solve a problem or challenge. The character's challenge is the one that creates tension throughout the story.
In most stories, there are 3 challenges. The number 3 is a mystical number symbolizing completeness. Try to come up with interesting challenges with which your character needs to struggle.
See a few examples below:
In the beginning of the story (or the exposition), you will need to introduce the setting and characters. You might also want to introduce the main conflict. This part of the story is important because it gives the reader necessary background information and maybe even a first insight into a character’s personality.
The setting (time & place) of a story can change throughout the plot.
Sensory details include sight, sound, touch, smell, and taste. These details are important because they create depth in your setting.
See a few examples below:
Characters are essential to a good story. Usually, the protagonist(s) is/are the most affected by the plot. Introduce a character by focusing on their actions, interests, and occupation, as the physical appearance doesn't make a difference in most cases.
Type in the name of your character.