por Alexander Ichine hace 1 año
173
Important Terms
por Alexander Ichine hace 1 año
173

Ver más
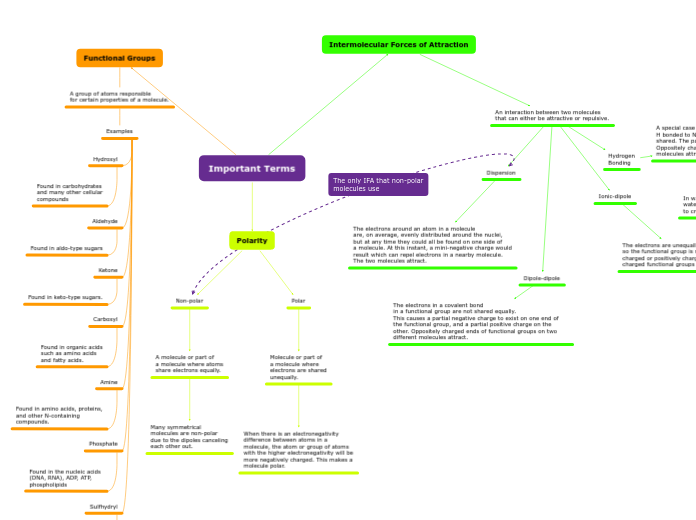
The electrons are unequally shared so the functional group is negatively charged or positively charged. Oppositely charged functional groups attract.
A special case of dipole involving functional groups containing H bonded to N or O because electrons are more unequally shared. The partial negative and positive charges are stronger. Oppositely charged ends of functional groups on two different molecules attract.
In water, hydrogen in one water molecule connect to another water molecule's oxygen to create a link of water molecules.
The electrons in a covalent bond in a functional group are not shared equally. This causes a partial negative charge to exist on one end of the functional group, and a partial positive charge on the other. Oppositely charged ends of functional groups on two different molecules attract.
The electrons around an atom in a molecule are, on average, evenly distributed around the nuclei, but at any time they could all be found on one side of a molecule. At this instant, a mini-negative charge would result which can repel electrons in a nearby molecule. The two molecules attract.
When there is an electronegativity difference between atoms in a molecule, the atom or group of atoms with the higher electronegativity will be more negatively charged. This makes a molecule polar.
Many symmetrical molecules are non-polar due to the dipoles canceling each other out.
Sulfhydryl
Found in the amino acid cysteine and thus in most proteins
Phosphate
Found in the nucleic acids (DNA, RNA), ADP, ATP, phospholipids
Amine
Found in amino acids, proteins, and other N-containing compounds.
Carboxyl
Found in organic acids such as amino acids and fatty acids.
Ketone
Found in keto-type sugars.
Aldehyde
Found in aldo-type sugars
Hydroxyl
Found in carbohydrates and many other cellular compounds