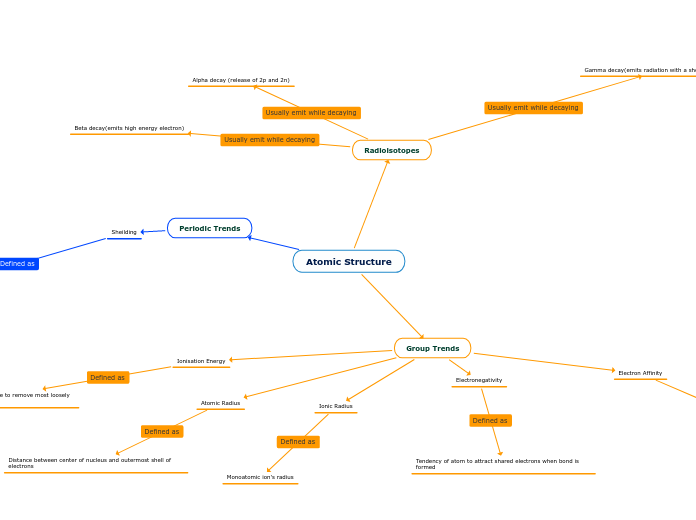
Atomic Structure
Group Trends
Ionisation Energy
Least amount of energy it would take to remove most loosely bonded electron
Atomic Radius
Distance between center of nucleus and outermost shell of electrons
Ionic Radius
Monoatomic ion's radius
Electron Affinity
Energy released when an electron is attached to a neutral atom
Electronegativity
Tendency of atom to attract shared electrons when bond is formed
Periodic Trends
Sheilding
Attraction between valance electrons and nucleus, as well as repulsion between electrons
Radioisotopes
Alpha decay (release of 2p and 2n)
Beta decay(emits high energy electron)
Gamma decay(emits radiation with a short wavelength