C1
C1.1
C1.1.1-Paritcles
Everything is made from matter
Matter is made from paritcles
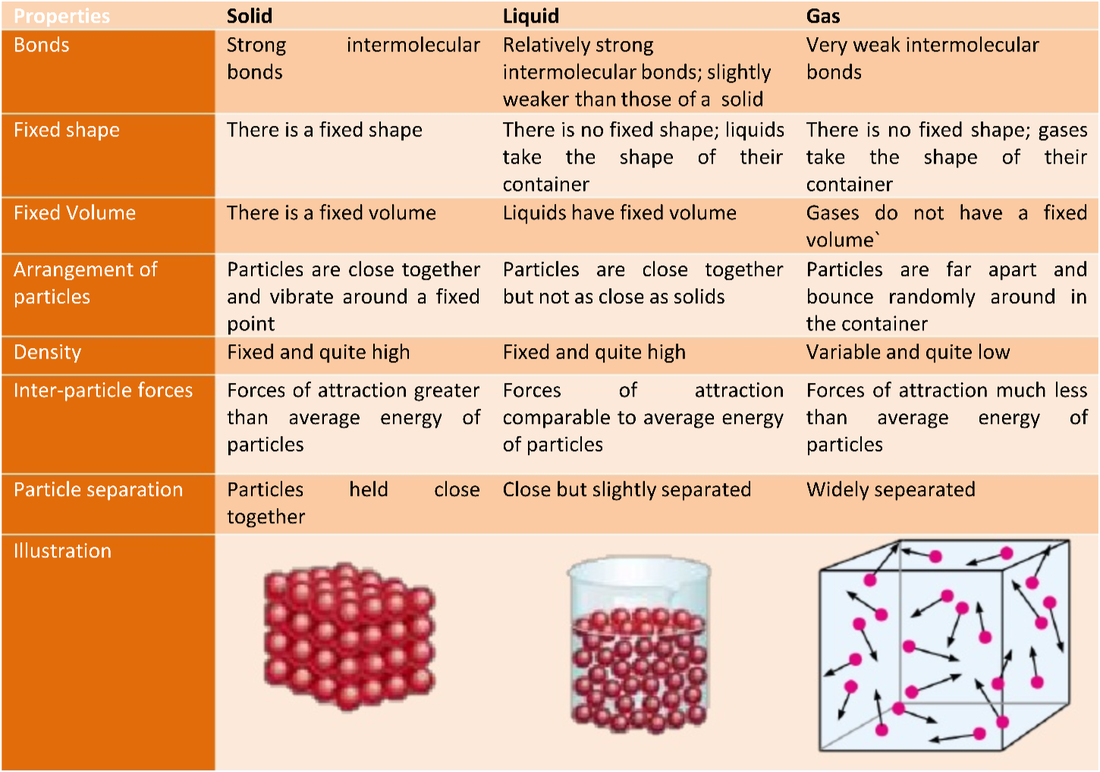
Models are used to solve problems, making predictions and developing scientific knowledge
The particle model is used to describe the arrangement of
particles and their different states
The particle model is used to explain certain properties
of a substance
C1.1.2-Chemical and physical changes
Physical changes is a change of state
E.g. Ice melting
Reversible and no new substances made
In a physical change the particles are the same
but their arrangement and movement change
Chemical change is a change which produces new substances
It usually irreversible and creates a new substance
The particles then form in a different way
C1.1.3-Limitations of the particle model
Size
The smallest particle that make up a substance
is called an atom
The smallest atoms are helium at 62x10^-12 m
in diameter
Distance between two helium is 55x larger than
diameter of the atom
Space
To calculate ratio of an atom
Ratio= Distance between atoms
Diameter of atom
Forces
Particles are held together by electrostatic forces
Forces of attraction between positive
and negative charges
The further the particles the weaker the forces
C1.2
C1.2.1-Atomic structures
Size of atoms
The size of an atom is given by its
atomic radius(half its diameter)
The length of the bond is the distance between
two joined atoms
Atomic radii and bond length is typically around
10^-10 (0.1nm)
Subtopic
Elements
Substances whose atoms have the same atoms
have the same atomic number