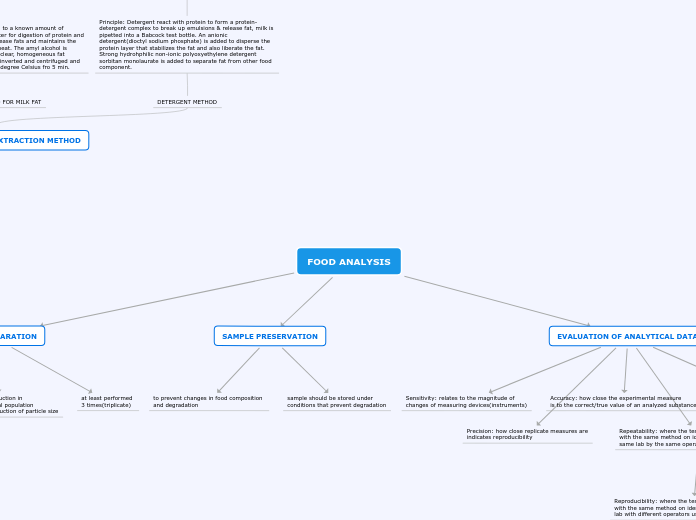
FOOD ANALYSIS
SAMPLE PREPARATION
depend on nature of food
analysis requires reduction in
amount from the total population
& simultaneously reduction of particle size
at least performed
3 times(triplicate)
SAMPLE PRESERVATION
to prevent changes in food composition
and degradation
sample should be stored under
conditions that prevent degradation
EVALUATION OF ANALYTICAL DATA
Sensitivity: relates to the magnitude of
changes of measuring devices(instruments)
Accuracy: how close the experimental measure
is to the correct/true value of an analyzed substance
Specificity: of a particular analytical method
(how well the method detects & measures the
compound of interest)
Precision: how close replicate measures are
indicates reproducibility
Repeatability: where the test results are obtained
with the same method on identical test items in the
same lab by the same operator within short interval time.
Reproducibility: where the test results are obtained
with the same method on identical test items in different
lab with different operators using different equipments.
MOISTURE
OVEN DRYING METHOD
PRINCIPLE: The sample is heated under specified
conditions until constant weight, and calculation of moisture is based on loss of weight and the thermal energy is used to evaporate the water from the food sample
ADVANTAGES: precise,relatively cheap,easy to use
officially approved for many samples can be analyzed.
DISADVANTAGES: destructive, unsuitable for some types of food and time consuming
DISTILLATION METHOD
PRINCIPLE: involves co-distilling the water in a
food sample with a high boiling point solvent that is
immiscible in water, the distilled water is condensed.
collecting the mixture that distills off in a collecting vessel
or trap- measuring the volume of H20
DIRECT DISTILLATION(DEAN AND STARK)
OR REFLUX DISTILLATION
ADVANTAGES: suitable for application to foods with low-
moisture contents, application to food containing volatile oils,
equipment is relatively cheap, easy to setup and operate.
DISADVANTAGES: destructive, relatively time-consuming, involving the use of flammable solvents, not applicable to some types of food.
CHEMICAL METHOD- KARL-FISCHER
TITRATION
PRINCIPLE: Karl-Fischer reagent is added directly
as the titrant if the water in the sample is accessible, if the
moisture is inaccessible to the reagent, the moisture is extracted from the food with an appropriate solvent.
A KFR water equivalent must be determined, iodine and SO2 in the appropriate form are added to the sample in a closed chamber protected from atmospheric moisture. The end point colour is dark-brown.
ADVANTAGES: suitable for low-moisture foods that
are sensitive to decomposition/ volatilization under vaccum
or high temperature.
DISADVANTAGES: incomplete water extraction due to atmospheric condition, moisture adhering to the walls of unit, interferences from certain food constituents.
CRUDE FAT
WET SOLVENT EXTRACTION METHOD
GOLDFISCH METHOD
Principle: Sample is put in an extraction ceramic
thimble and the solvent is added into the boiling flask.
The sample must ground to small particle size prior to analysis. Method allows the solvent from boiling flask to continuously flows over the sample that held in a ceramic thimble.
ADVANTAGE: faster and more efficient extraction
method than Soxhlet extraction method.
DISADVANTAGE: channeling of the solvent may occur inefficient/ incomplete extraction.
SOXHLET METHOD
Principle: Thimble(containing the sample) is placed
in an extraction chamber, which suspended above a flask containing the solvent & below the condenser. The flask is heated, the solvent evaporates & moves up into the condenser and converted into a liquid & drips back into the extraction chamber containing sample.
ADVANTAGE: provides soaking effect of the sample(provide more complete extraction of fat from the sample), avoid channeling of the solvent.
DISADVANTAGE: the extraction took a long time(4 hours- 16 hours)
MOJONNIER METHOD
Principle: Fat is extracted with a mixture of ethyl ether
and petroleum-ether in a Mojonnier flask. The extracted fat is dried to a constant weight and expressed as percent(%) fat by weight.
ADVANTAGE: does not require prior removal of moisture from
sample, extraction are repeated three times
DISADVANTAGE: use many chemical reagents during the extraction process.
NON- WET SOLVENT EXTRACTION METHOD
BABCOCK METHOD FOR MILK FAT
Principle: Sulphuric acid is added to a known amount
of milk in Babcock bottle. The mixture is shaken until homogeneous,centrifuged and submerged into water at 63 degree Celsius. Subsequently centrifugation and addition of hot water isolate fat for quantification in the graduated portion of test bottle.
ADVANTAGE: for modified Babcock method it can be used
to determine essential oil in flavour extracts and fat in seafood.
DISADVANTAGE: it does not determine phospholipids in the milk products and not applicable to product containing chocolate or sugars.
GERBER METHOD FOR MILK FAT
Principle: Sulphuric acid is added to a known amount of
milk in Gerber tube or butyrometer for digestion of protein and carbohydrates- it also help to release fats and maintains the fat in liquid state by generating heat. The amyl alcohol is added into the mixture to give a clear, homogeneous fat column and the tube is carefully inverted and centrifuged and incubated in waterbath at 60-63 degree Celsius fro 5 min.
ADVANTAGE: wider application to a variety of dairy
products, it much simpler and faster because isoamyl alcohol generally improves the fat separation and reduces the effect of sulfuric acid and also prevent the charring of sugar.
DETERGENT METHOD
Principle: Detergent react with protein to form a protein-
detergent complex to break up emulsions & release fat, milk is pipetted into a Babcock test bottle. An anionic detergent(dioctyl sodium phosphate) is added to disperse the protein layer that stabilizes the fat and also liberate the fat. Strong hydrohphilic non-ionic polyoxyethylene detergent sorbitan monolaurate is added to separate fat from other food component.
Advantage: does not used corrosive reagent like in Babcock Method
PROTEIN
BIURET METHOD
Principle: Biuret method involves a reaction with peptide
linkages,cupric ions complexed with peptide bonds-under
alkaline conditions and produced a violet-purplish colour. Absorbance of the colour produced is read at 540nm. The colour intensity(absorbance) is proportional to the protein content of the sample.
Advantages: rapid test(analysis can be completed within 30 minutes), does not detect nitrogen from non-peptide or non-protein source.
Disadvantages: relatively low sensitivity compared to other UV-vis methods, opalescence could occur in the final solution with presence of high levels of lipids and carbohydrates.
DYE BINDING METHOD
Principle: Protein containing sample is mixed with a known
excess amount of anionic(negatively charged) dye in a buffered solution; proteins bind to the dye, to form an insoluble complex(electrostatic attraction between the molecule), the amount of unbound soluble dye is determined by measuring its absorbance. Unbound dye is inversely related to the protein content of the sample.
Advantage: rapid, inexpensive, relatively accurate,
no corrosive reagents,more precise than Kjedahl method, does not measure non-protein nitrogen.
Disadvantage: not sensitive; mg quantities of protein are required, proteins differ in basic amino acid content, so differ in dye-binding capacity, non-protein component bind dye until cause error.
LOWRY METHOD
Principle: Lowry method combines Biuret reagent with another
reagent (FOLIN-CIOCALTEAU PHENOL reagent) which reacts with tyrosine and trytophan residues in proteins. The reaction gives a bluish colour; the absorbance is read at 750nm( high sensitivity for low protein) 500nm( low sensitivity for high protein concentration)
Advantages: very sensitive, less affected by turbidity of the
sample, relatively simple
Disadvantage: colour varies with different protein to a
greater extent than Biuret method, colour is not strictly proportional to protein concentration. The reaction is interfered with varying degrees of sucrose,lipids,monosaccharides and etc.
KJEDAHL METHOD
Principle: Proteins and other organic food components
in a sample are digested with sulphuric acid in the presence
of catalysis. Total organic Nitrogen is converted to ammonium
sulfate; digest is neutralized with alkali and distilled into boric acid solution. Borate anions are formed & titrated with standardized acid- converted to nitrogen in the sample.
Advantages: applicable to all types of food, relatively simple and inexpensive.
Disadvantages: does not give a measure of the true
protein- measures total organic nitrogen, different proteins
need different correction factors, time consuming and use corrosive reagents.
CARBOHYDRATE
LANE-EYNON METHOD
Principle: Based on the reaction of reducing sugar
with a solution of copper sulfate followed by reaction
with alkaline tartrate. The mixture is boiled for a specific time,
followed by addition of methylene blue(as an indicator), coloured solution is titrated until decolouration of the indicator.
Advantage: for determinations of reducing sugars in
honey and other high reducing sugar syrups.
Disadvantage: The reaction is not stoichiometric-
necessary to prepare a calibration curve with a series of
standard solutions of known CHO concentration, result depends on the precise reaction time, temperature & reagent concentration), cannot distinguish between different types of reducing sugar.
MUNSON-WALKER METHOD
Principle: Involving oxidation of the CHO in the presence
of heat and an excess of copper sulfate and alkaline tartrate
under carefully controlled conditions-leads to the formation of a copper oxide precipitate. Amount of precipitate formed is directly related to the concentration of reducing sugar in the sample. Concentration of precipitate present can be determined through gravimetrically and titrimetrically.
Advantage: more reproducible and accurate
Disadvantage: same disadvantage as Lane-Eynon method
NELSON-SOMOGYI METHOD
Principle: The reducing sugar when heated with
alkaline copper tartrate reduce the copper, from
cupric to cuprous state, thus cuprous oxide is formed.
Cuprous oxide is treated with arsenomolybdate. Reduction
of arsenomolybdate complex produce an intense, stable
blue-coloured solution. The absorbance of the solution is
determined at either 500-520nm against standard
POLARIMETRY
Principle: Asymmetric carbon atoms have the
ability to rotate plane of polarization of polarized light.
Plane polarized light passed through solution exhibiting optical
activity, it rotated either to left(-) or right(+). Angle of polarization proportional to the concentration of optically
active molecules in solution. Prior to analysis, sample solution must be clarified. CHO able rotate plane polarized light through an angle of rotation.
Disadvantage: Polarimetry method unable to analyzed
mixtures of CHO(except sucrose in the presence of other CHO)
REFRACTIVE INDEX
(RI) MEASUREMENT
Principle: RI(n) of a substance is the ratio of light
velocity in a vacuum to its velocity of a substance.
When electromagnetic radiation passes from one medium
to another, it can change direction; RI of a material can be
determined by measuring the ratio of angle of incidence.
Advantage: Method is quick & simple to carry
out, gives direct reading and require only one or two
drops of sample, performed with simple hand-held instrument.
DIETARY FIBER
AOAC METHOD
Principle: to isolate the fraction of interest
by selective precipitation and then to determine
its mass by weighing. Insoluble fiber is collected by
filtration soluble fiber is precipitated by bringing the filtrate to
78% ethanol and collected by filtration.
Advantage: The method can be used to determine
fiber content in all foods
Disadvantage: greatly overestimate he fiber content with a
high content of simple sugars.
ACID & ALKALI DIGESTION METHOD
Principle: Digestible CHO, lipids and protein are selectively solubilized by chemical and enzymes. Indigestible materials are then collected by filtration, and the fibre residue is quantitated gravimetrically. This method only measures celluloses and lignin.
Disadvantage: The method measures variable amounts
of the celluloses and lignin in the sample, but hemicelluloses, pectins and hydrocolloids are solubilized and not detected.
THEANDER-MARLETT METHOD
Principle: free sugars and lipids are extracted with ethanol
and hexane. Starch is removed by enzymatic digestion & insoluble fiber is separated from soluble fiber. Fiber fractions are hydrolyzed with sulfuric acid and sugar content of the acid hydrolysates is determined. Lignin is determined gravimetrically.
Advantage: suitable for research, legislation, and labeling purpose, provides most accurate estimate of fiber for wide range of food.
ENGLYST-CUMMINGS METHOD
Defatted food sample is heated in water. Enzymes are then
added to precipitate fiber, which is separated from digest by
centrifugation, then is washed & dried. Fiber is then hydrolyzed using concentrated sulfuric acid solution to break down into mono. Concentration of mono is determined colorimetrically or chromatographically. Mass of fiber in original sample assumed to be equal to the total mono present.
Advantage: suitable for determining fiber content
in most foods(low content of ligning), it allow estimation
of resistant starch.
ASH AND MINERALS
ALKALINITY OF ASH
PROCEDURE :Place ash (total / water-insoluble ash) in platinum dish. Add 0.1N HCl and warm on a steam bath.
Cool and transfer to Erlenmeyer flask, titrate HCl with 0.1N NaOH using methyl orange as indicator.
Express the result as mL of 1N acid / 100g sample.
ACID INSOLUBLE ASH
PROCEDURE: Ash is diluted with distilled water, then heated to nearly boiling, the resulting solution is filtered and washed several times with hot distilled water.
Dry and re-ash the filter paper in muffle furnace at least 30 min. until constant weight is achieved. The weight remaining represents the amount of insoluble ash.
Calculate soluble ash by subtracting insoluble ash from total ash, or, dry the filtrate, re-ash and weigh.
LOW TEMPERATURE PLASMA
DRY ASHING
PRINCIPLE: Sample is placed into a glass chamber, sealed and vacuum is applied.
Small flow of O2 / air is introduced into the system while maintaining the specific minimum vacuum. Electromagnetic radio frequency generator is activated to control the rate of incineration, excites the gas molecules and dissociates it into chemically active atoms and molecules. Combustion products which are completely dissociated are carried away in the gas stream.
Variable power frequency adjusts the rate of incineration.
ADVANTAGE:Less chances of losing trace elements by volatilization, Equipment of choice for volatile salts.
Utilization of O2 as sole reagent.
Disadvantage: small capacity and the equipment is expensive.
DRY ASHING
PRINCIPLE:. Incineration at high temp. with muffle furnace (5250 degree celcius or higher)
ii. Water & other volatile materials are vaporized, organic substances are burned in the presence of the O2 in air to CO2, H2O and N2.
iii.Most minerals are converted to oxides, sulfates, phosphates, chlorides or silicates.
iv.The food sample is weighed before and after ashing to determine the concentration ash present.
ADVANTAGE:Safe method,Requires no added reagents or blank subtraction. Large number of crucibles can be handled at once.Resultant ash can be used for other analyses e.g. acid insoluble ash, and water soluble and insoluble ash, Requires little attention, not labour intensive.
DISADVANTAGE:Time consuming (12 – 18 hrs, or overnight) Loss of volatile elements at high temp. e.g. Cu, Fe, Pb, Hg, Ni, Zn, Interactions between mineral components and crucibles.
WET ASHING
PRINCIPLE: Oxidation of organic substances by strong acid (HNO3) and oxidizing agent, perchloric acid (HClO4), Sample solution is heated up slowly up to 350 degree celsius until organic matter is completely digested (leaving only mineral oxides in solution) and HNO3 is almost evaporated, Boiling of sample solution is continued until the solution become colourless or light in colour, Solution is cooled, and 50% of HCl is added and diluted with distilled, deionized water.
Advantage: mineral stay in solution,little loss from mineral volatilization, rapid than dry ashing.
Disadvantage: hazardous and used corrosive reagent.
VITAMINS
METHODS
COLORIMETRIC METHODS- MEASURES THE UNSTABLE
COLOUR AT A620NM THAT RESULTS FROM REACTION
BETWEEN VIT A AND ANTIMONY TRICHLORIDE. BLUE COLOUR= AMOUNT OF RETINOL IN FOOD SAMPLE. THE COLOUR IS MEASURED AGAINST THE SE OF KNOWN STANDARDS.
HPL METHOD- THIS METHOD INVOLVES CHROMATOGRAPHIC SEPARATION AND QUANTITATIVE DETERMINATION AT 325NM.
METHODS
2,6-DICHLOROPHENOLINDOPHENOL TITRIMETRIC METHOD:
L-ASCORBIC ACID IS OXIDIZES TO DEHYDROASCORBIC ACID BY INDICATOR DYE. IT MEASURES THE DECOLOURIZATION OF DYE BY ASCORBIC ACID. AT THE ENDPOINT, EXCESS OF UNREDUCED DYE IS ROSE PINK IN ACID SOLUTION LASTING AT LEAST 10 SEC.
DISADVANTAGE: THIS METHOD IS NOT SUITABLE
FOR HIGHLY COLOURED PRODUCTS BECAUSE OF
DIFFICULTY OF DETERMINING THE ENDPOINT DURING
TITRATION.
FLUOROMETIC METHOD: THIS METHOD MEASURES BOTH ASCORBIC ACID AND DEHYDROASCORBIC ACID. ASCORBIC ACID OXIDIZED TO DEHYDROASCORBIC ACID UPON REACTION WITH O-PHENYLAMINE. THE REACTION WILL FORMED A FLUORESCENT QUINOXALINE COMPOUND. THE FLUOROSCENT COMPOUND= VIT. C CONTENT.
METHOD
COLORIMETRIC METHOD: REACTION BETWEEN NIACIN(NICOTIC ACID AND CYNOGEN BROMIDE WILL FORMS COLOURED COMPOUND WITH AN INTENSITY PROPORTIONAL TO NIACIN CONCENTRATION.
DISADVANTAGE: TOXICITY OF CYNOGEN BROMIDE- THE ANALYSIS MUST BE CARRIED OUT UNDER FUME HOOD.
METHOD
THIOCHROME FLUOROMETRIC METHOD: THE MATERIAL TO BE EXAMINED MUST DIGESTED WITH SULFURIC ACID AND TREATED WITH A PHOSPHATASES PREPARATION. THE THIOCHROME RESULTING FROM OXIDATION WITH POTASSIUM FERRICYANIDE, HYDROGEN PEROXIDE IN ALKALINE SOLUTION IS EXTRACTED WITH ISOBUTYL ALCOHOL.
THE INTENSITY OF THE BLUE FLUORESCENCE PROPORTIONAL TO THE THIAMINE CONCENTRATION. AND IT IS COMPARED WITH STANDARD SOLUTION.