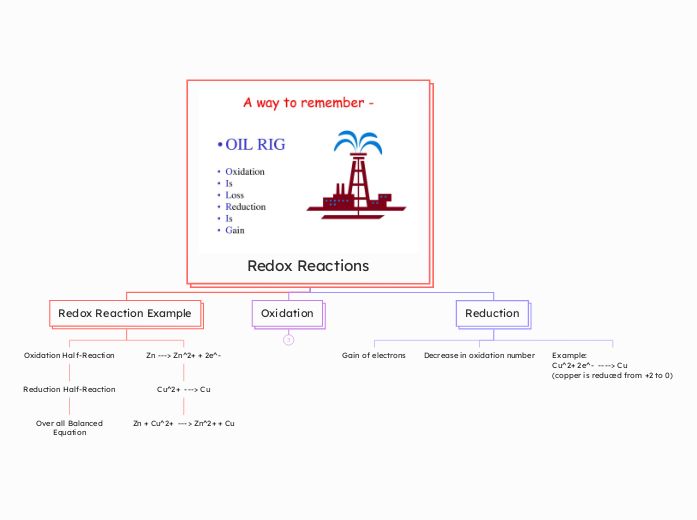
Redox Reactions
Redox Reaction Example
Oxidation Half-Reaction
Reduction Half-Reaction
Over all Balanced
Equation
Zn ---> Zn^2+ + 2e^-
Cu^2+ ---> Cu
Zn + Cu^2+ ---> Zn^2+ + Cu
Oxidation
Reduction
Gain of electrons
Decrease in oxidation number
Example:
Cu^2+ 2e^- ----> Cu
(copper is reduced from +2 to 0)