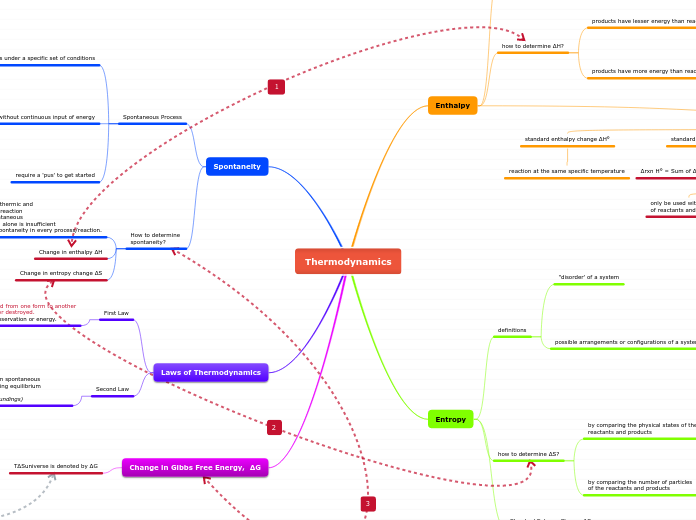
Thermodynamics
Enthalpy
definition
Enthalpy change ΔH
- amount of heat released or absorbed
by a chemical system when a chemical
reaction occurs at constant pressure.
how to determine ΔH?
products have lesser energy than reactants
Heat released to
surroundings
-ve ΔH
exothermic reaction
products have more energy than reactants
Heat absorbed to
surroundings
+ve ΔH
endothermic reaction
Standard Enthalpy Change
standard enthalpy change ΔH°
reaction at the same specific temperature
standard enthalpy of reaction, Δrxn H°
∆rxn H° = Sum of Δf H° (product) - Sum of Δf H° (reactant)
only be used with the Δf H°
of reactants and products
unit of Δrxn H° is kJ
standard enthalpy change
of formation, Δf H°
one mole of compound is formed from
its element in their reference states
Δf H° of an element &
7 diatomic elements
(H2, N2, O2, F2, Cl2, Br2, l2)
Δf H° = 0 kJ/mol
standard bond dissociation
enthalpy
bond breaking
energy is absorbed
endothermic reaction
ΔH bond breaking is +ve
bond forming
energy released
exothermic reaction
ΔH bond forming is -ve
Entropy
definitions
"disorder' of a system
possible arrangements or configurations of a system
Examples
most number of possible outcomes
gaseous state
least number of possible outcomes
solid state
how to determine ΔS?
by comparing the physical states of the
reactants and products
solid to gas
increase in the number of possible arrangements
gas to solid
decrease in the number of possible arrangements
by comparing the number of particles
of the reactants and products
increase in the number of particles
increase in the number of possible arrangements
decrease in the number of particles
decrease in the number of possible arrangements
Standard Entropy Change ΔS
∆rxnS = S (product) - S (reactant)
Spontaneity
Spontaneous Process
occurs under a specific set of conditions
occurs without continuous input of energy
Examples
non-spontaneous
water freezing at 25 °C 1 atm
spontaneous
ball moving down a slope
require a 'pus' to get started
How to determine
spontaneity?
- both endothermic and
exothermic reaction
can be spontaneous
- sign of ∆H alone is insufficient
to predict spontaneity in every process/reaction.
Change in enthalpy ΔH
Change in entropy change ΔS
Laws of Thermodynamics
First Law
energy can be converted from one form to another
but cannot be created or destroyed.
Based on the law of conservation or energy.
Second Law
the entropy of the universe increases in spontaneous processes and remains unchanged during equilibrium processes.
(∆S universe = ∆S system + ∆S surroundings)
equilibrium process,
∆S universe = 0
spontaneous process,
∆S universe = + ve
ΔG = ∆H system - T∆S system
Change in Gibbs Free Energy, ΔG
TΔSuniverse is denoted by ΔG