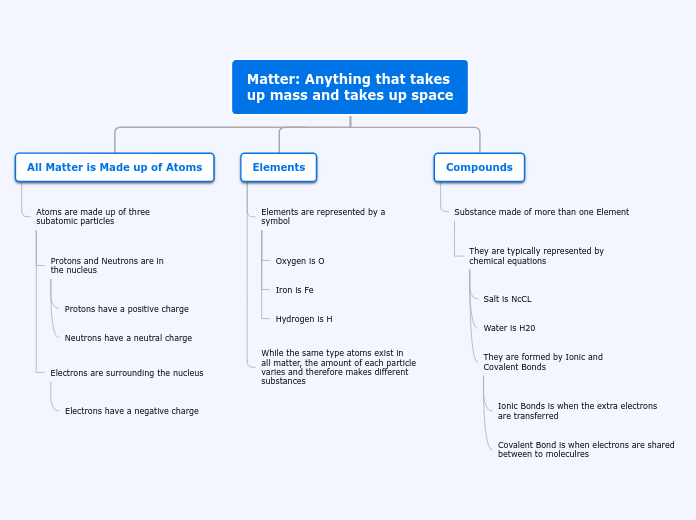
Matter: Anything that takes
up mass and takes up space
All Matter is Made up of Atoms
Atoms are made up of three
subatomic particles
Protons and Neutrons are in
the nucleus
Protons have a positive charge
Neutrons have a neutral charge
Electrons are surrounding the nucleus
Electrons have a negative charge
Elements
Elements are represented by a
symbol
Oxygen is O
Iron is Fe
Hydrogen is H
While the same type atoms exist in
all matter, the amount of each particle
varies and therefore makes different
substances
Compounds
Substance made of more than one Element
They are typically represented by
chemical equations
Salt is NcCL
Water is H20
They are formed by Ionic and
Covalent Bonds
Ionic Bonds is when the extra electrons
are transferred
Covalent Bond is when electrons are shared
between to moleculres