arabera Bajwa Quintin 4 years ago
346
Organic Nomenclature
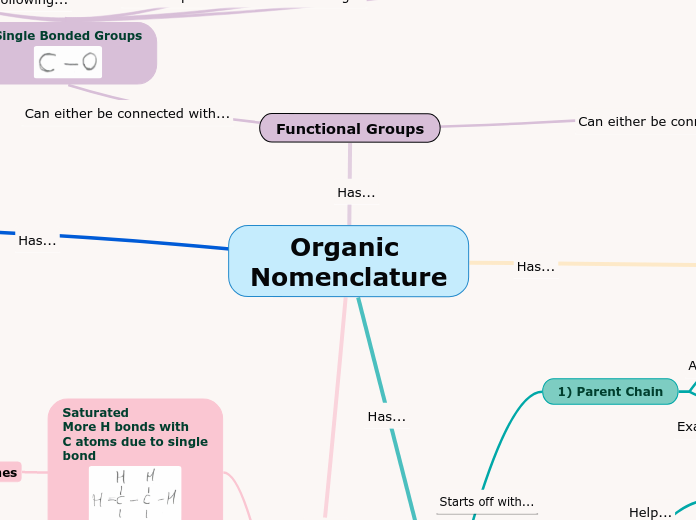
arabera Bajwa Quintin 4 years ago
346

Honelako gehiago
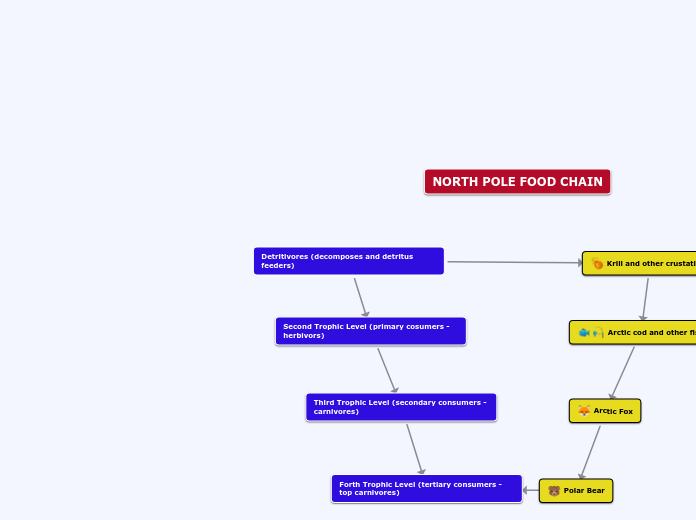

catherine patterson‑k egina


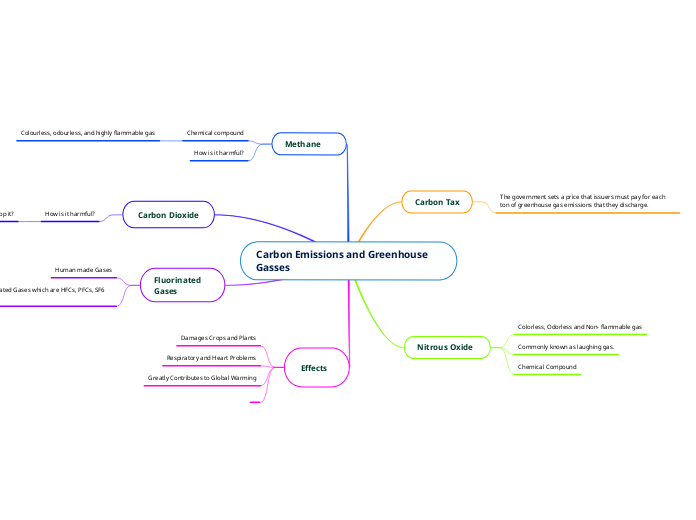
maria garcia‑k egina

HI - 07MA 808655 Roberta Bondar PS‑k egina
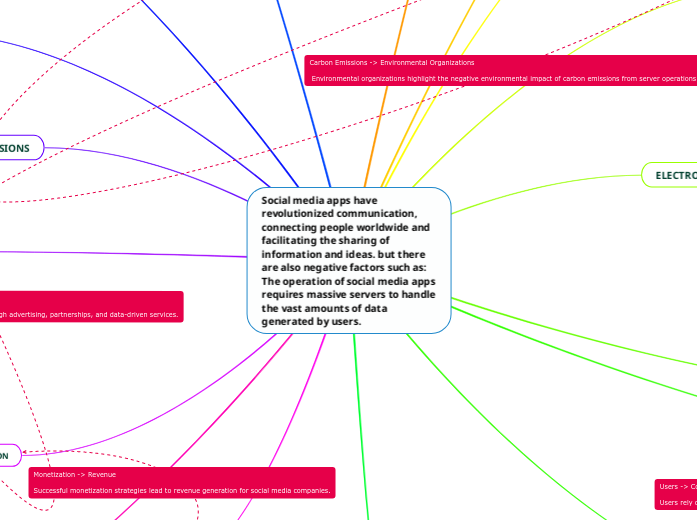

Huzaifa Ahmed‑k egina
Number of carbons that aren't connected to the parent chain
Trans-2-butene
Cis-2-butene
Suffix: -one
Butanone
Suffix: -amide
Methanamide
Suffix: -al
Methanal
Esters
Suffix: -oate
Propyl Ethanoate
Suffix: -oic acid
Propanioic Acid
Propan-1-amine
2-ethoxypropane
Trichloromethane
Tertiary The carbon with the hydroxyl is bonded to three other carbon
C has no H bonds and 3 R bonds
Secondary The carbon with the hydroxyl is bonded to two other carbon
C has 1 H bond and 2 R bonds
Primary The carbon with the hydroxyl is bonded to one other carbon
C has 2 H bonds and 1 R bond
Multiple -OHs Diol, Triol, Tetra
Ethane-1,1-diol
Suffix: -ol
Ethanol
[
When water splits a bond into two
Two organic molecules join to form one organic molecule with water molecule
Reverse of addition where an atom is removed to from a double bond
When a functional groupe is replaced with another group
When a reactant is oxidized an another is reduced
Creates more bonds with H and fewer with O
When atoms are added to form a double or triple bond