Intermolecular Forces
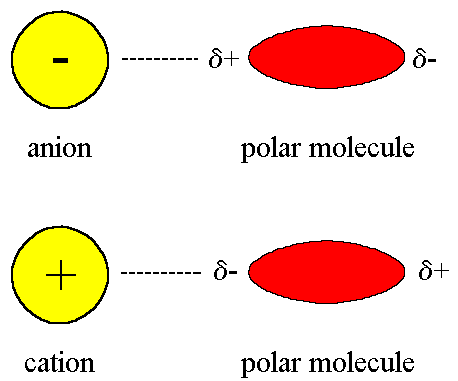
Ion-Dipole
The electrostatic interaction between a charged ion and a neutral molecule that has a dipole
Most commonly found in solutions
Attraction becomes stronger as either the charge on the ion increases, or as the magnitude of the dipole of the polar molecule increases
Example:

In this example, the oxygen atom in the water molecule has a slightly negative charge and is attracted to the positive sodium ion.
Dipole-Dipole
The force of attraction between the slightly positive end of one polar molecule and the slightly negative end of an adjacent polar molecule
Occurs between neutral polar molecules
Weaker than ion-dipole forces
Increase with an increase in the polarity of the molecule
Only has a significant effect when the molecules involved are close together
Example:

In this example, the partial negative charge of the chlorine atom attracts to the partial positive charge of the hydrogen atom of the other compound.
If the dipoles of a molecule cancel each other out, then the molecule is non-polar and symmetrical. If not, then the molecule is polar and asymmetrical
Induced Dipole-Dipole
The force of attraction that forms when a polar molecule induces a dipole in a non-polar molecule by disturbing the arrangement of electrons
A weak form of attraction

Example:

In this example, the water molecule (polar) induces a dipole into the oxygen molecule (non-polar) which results in a weak attraction between the two.
Hydrogen Bonding
The force of attraction between a hydrogen atom attached to a highly electronegative atom (N, O, or F) and a highly electronegative atom in another molecule
Dipole-dipole interaction
Positive charge is attracted to the negative charge of an electronegative atom in a nearby molecule
Stronger than dipole-dipole or dispersion forces
Important in the organization of biological molecules
Example:
In this example, the partially negative oxygen atom in one of the water molecules is attracted to the partially positive hydrogen atom in another nearby water molecule
London Dispersion
The force of attraction acting between all entities, including polar molecules, non-polar molecules and unbonded atoms, caused by the temporary imbalance of electrons within the entities
Temporary dipole induces a dipole into neighboring molecules
Weakest intermolecular force
The greater the polarizability, the stronger the dispersion forces
Larger the molecule, the greater the polarizability
Example:
In this example, the uneven distribution of electrons in the He atom (left) causes a temporary dipole (middle). This induces a dipole in the neighboring He atom (right).
The set of attractive and repulsive forces that occur between molecules
They strength of the intermolecular forces determines the following physical properties of molecular compounds:
Physical state of a compound at a specific temperature and pressure
Melting Point
Boiling Point
Surface Tension
Hardness and Texture
Solubility in various solvents
