Water Pollution: Everything You Need to Know
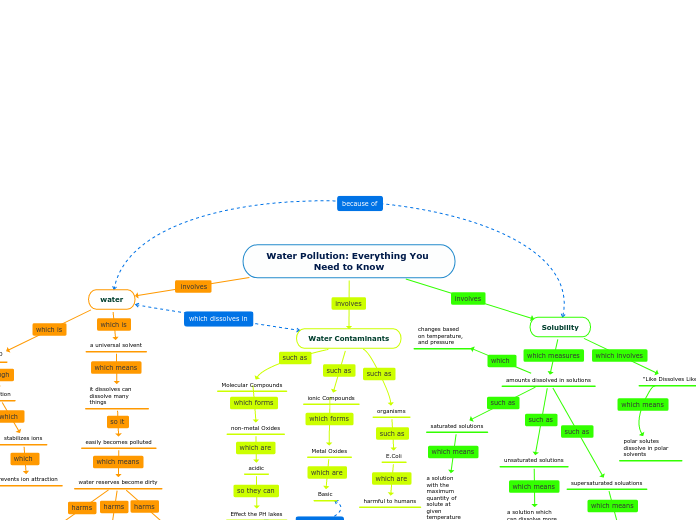
water
H2O
hydration
Separate Ions
Dissociation
stabilizes ions
prevents ion attraction
a universal solvent
it dissolves can dissolve many things
easily becomes polluted
water reserves become dirty
humans
aquatic life
ecosystems
Water Contaminants
ionic Compounds
Metal Oxides
Basic
Molecular Compounds
non-metal Oxides
acidic
Effect the PH lakes
kill ecosystems
organisms
E.Coli
harmful to humans
Solubility
amounts dissolved in solutions
changes based on temperature, and pressure
saturated solutions
a solution with the maximum quantity of solute at given temperature and pressure
unsaturated solutions
a solution which can dissolve more solute at given temperature and pressure
supersaturated soluations
a solution that contains more than the maximum quantity of solute at given temperature and pressure
"Like Dissolves Like"
polar solutes dissolve in polar solvents
non-polar solutes dissolve in non-polar solvents