par Rohan Malvi Il y a 5 années
623
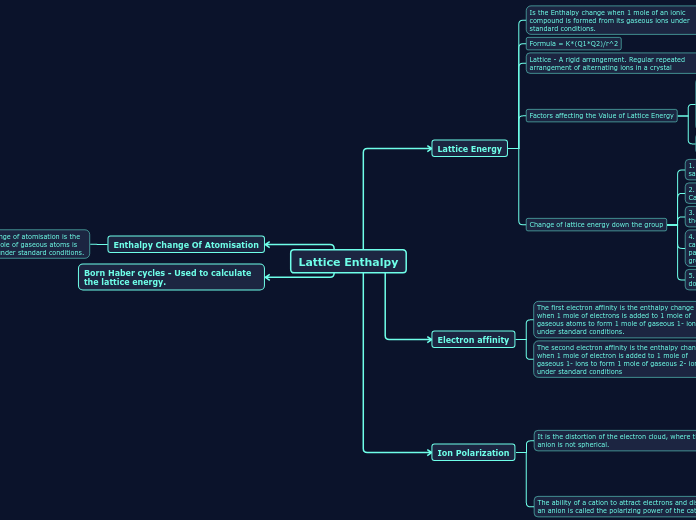
Lattice Enthalpy
par Rohan Malvi Il y a 5 années
623

Plus de détails
4. The anion has a charge of 2- or 3-
3. Anion is large
2. Cation has a charge of 2+ or 3+
1. Cation is small
2. The ease with which the anion can be polarized (Polarizabilty)
1. The charge density of the cation