Evolution of the Atom
By: Kevin Do
Early Greeks
~400 BCE
Aristotle
Thought all matter was made out of 5 elements: Earth, fire, air, water and ether.
Democritus
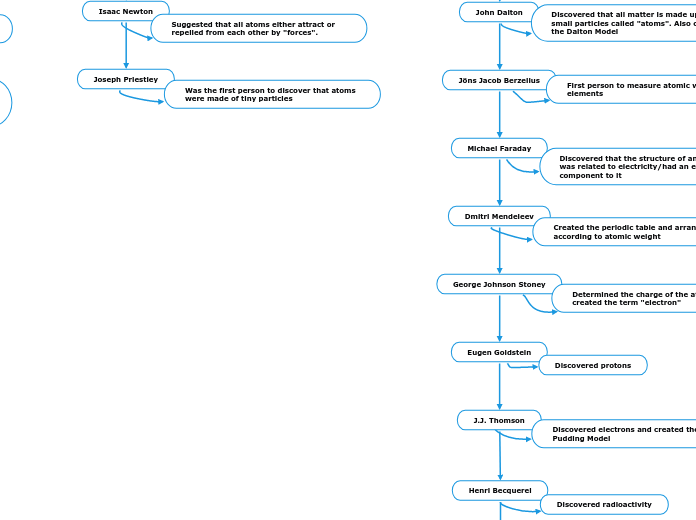
1700's
Isaac Newton
Suggested that all atoms either attract or repelled from each other by "forces".
Joseph Priestley
Was the first person to discover that atoms were made of tiny particles
1800's
John Dalton
Discovered that all matter is made up of small particles called "atoms". Also created the Dalton Model
Jöns Jacob Berzelius
First person to measure atomic weights for elements
Michael Faraday
Discovered that the structure of an atom was related to electricity/had an electric component to it
Dmitri Mendeleev
Created the periodic table and arranged it according to atomic weight
George Johnson Stoney
Determined the charge of the atom and created the term "electron"
Eugen Goldstein
Discovered protons
J.J. Thomson
Discovered electrons and created the Plum Pudding Model
Henri Becquerel
Discovered radioactivity
Marie and Pierre Curie
Discovered Polonium, Radium and radioactivity originates
within the atom itself.
1900's
Max Planack
Theorized that matter emits/absorbs
energy in small packets of energy called quantum.
Nagaoka Hantaro
Created the Saturnian model.
Albert Einstien
Confirmed the existence of atoms and molecules using mathematical calculations.
Robert Millikan
Ernest Rutherford
Neils Bohr
Modified the Rutherford Model to create the Bohr Model and discovered that electrons orbit the nucleus of fixed sizes and energies
Louis De Broglie
Introduced the idea that particles have wave like properties
Erwin Schrodinger
Created the Schrödinger wave equation
Paul Dirac
Created a fully relativistic quantum theory.
James Chadwick
Discovered the neutron
Discovered the nucleus of an atom and created the Rutherford Atomic Model
Discovered the fact that all electrons carry the same amount of negative charge