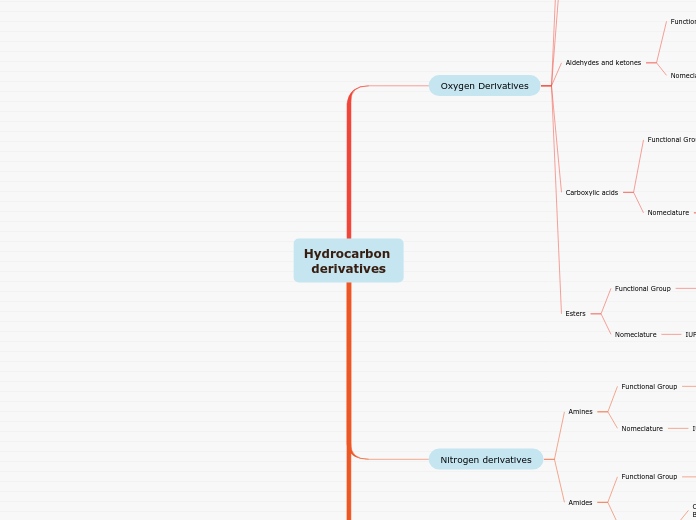
Hydrocarbon derivatives
Oxygen Derivatives
Alcohols
Functional Group
Hydroxyl
(R-OH)
Nomlecature
IUPAC
CH3-CH2-OH
ethanol
CH3-CH-CH3
|
OH
2-propanol
Ethers
Functional Group
R-O-R
Nomeclature
IUPAC
CH3-O-CH2-CH3
ethyl methyl ether
. CH3
|
H3C-C-O-CH3
|
CH3
Tertbutyl methyl ether
Aldehydes and ketones
Functional Group
Carbonyl
(R-C=O)
Nomeclature
IUPAC
CH2=O
Methanal
Formaldehyde
CH3-CH-CH3
||
O
Propanone
Dimethyl ketone
Carboxylic acids
Functional Group
Carboxyl
(R-C=O, R-COOH, R-CO2H)
|
OH
Nomeclature
IUPAC
CH3-CH2-CH2-COOH
Butanoic acid
CH3(CH2)8COOH
Decanoic acid
Common name
CH3-CH2-CH2-COOH
Butyric acid
CH3(CH2)8COOH
Capric acid
Esters
Functional Group
Combination of carboxyl and hydroxyl
(R-COO-R, R-C-O-R)
||
O
Nomeclature
IUPAC
CH3-COO-(CH2)7-CH3
Octyl acetate
CH3-CH2-CH2-COO-CH2-CH3
Ethyl butyrate
Nitrogen derivatives
Amines
Functional Group
R-N-H, R-N-R', R-N-R'
| | |
H H R''
Nomeclature
IUPAC
CH3-NH-CH3
Dimethylamine
CH3-CH2-NH-CH3
Ethylmethylamine
Amides
Functional Group
Amino group (-NH2) replaces -OH
(R-C-NH2, R-CO-NH3)
|
O
Nomeclature
CH3-CH2-CH2-CO-NH2
Butanoamide
CH3-CH2-CO-NH2
Propionamide
Halogen derivatives
Functional Group
Monohalogenated
(R-X where R represents the hydrocarbon chain and X any of the halogens)
Nomeclature
IUPAC
CH3-CH2-CH2-CH3-Br
BUtyl bromide
CH2-CH-CH2
| | |
Cl Cl Br
3-bromo-1,2-dichloropropane
Common
CH3-Cl
Methyl chloride
CHCl3
Chloroform