Solvent-Free Synthesis
Solvent Synthesis
Reacting products in a solvent
Produces a great amount of waste
Due to creating waste it is often inefficient
Pollutive
Solid state synthesis
Reacting products without a medium (solvent).
Most organic reactions occur in presence of a solvent, thus creating the theory that every reaction must occur in a solution.
Historically, this theory was used to preserve food product, remove the median of the reaction thus slowing it down considerably.
E.G
Carboxymethyl Cellulose (CMC)
physiologically active 2-amino-4H-Benzo[b]pyrans 8
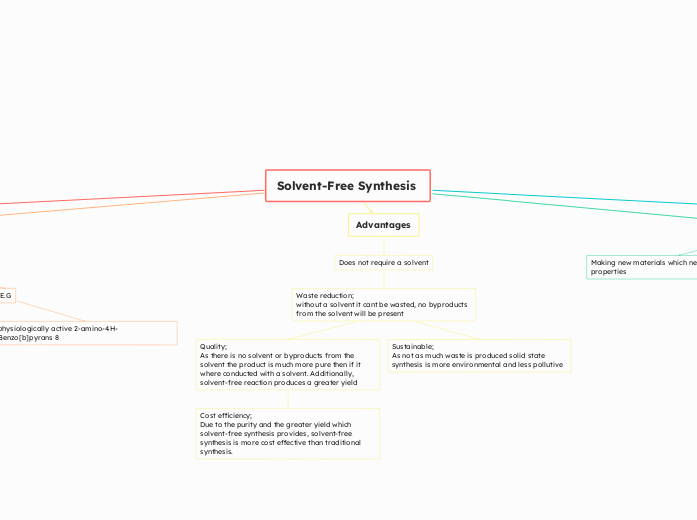
Advantages
Does not require a solvent
Waste reduction;
without a solvent it cant be wasted, no byproducts from the solvent will be present
Quality;
As there is no solvent or byproducts from the solvent the product is much more pure then if it where conducted with a solvent. Additionally, solvent-free reaction produces a greater yield
Cost efficiency;
Due to the purity and the greater yield which solvent-free synthesis provides, solvent-free synthesis is more cost effective than traditional synthesis.
Sustainable;
As not as much waste is produced solid state synthesis is more environmental and less pollutive
Application
Making new materials which new or different properties
Making the chemical synthesis industry produce less pollution and more cost effective.
Limitations
Difficult to control the reaction.
Only achievable on small scales
Reaction only achievable by mechanical processes
I.e. Grinding (mortar and pistil), thermal heating, microwaves.