Gum and Stabilizers
-A range of polysaccharides and protein.
-Usage level <2%
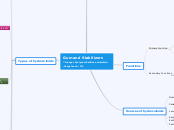
Types of hydrocolloids
Cellulose derivates
Batter
-Retard loss of moisture and improve adhesion
Fried foods
-Create a barrier to oil absorptio
Functions:
-thickening, suspending, stabilizing & modify flow characteristics
Optimum pH range: 4-10
Chemically modified cellulose into carboxymethylcellulose (CMC), hydroxypropylmethycellulose (HPMC) and methylcellulose (MC)
Xanthan gum
Functions:
-Thickening, suspending, and stabilizing effect
Optimum pH range: 1-13
Optimum soluble solids range: 0-80%
Solubility in water: soluble in cold and hot water
Gelling conditione: Gel at temperature below setting temperature
Polysaccharides produced from fermentation of CHO substrate with xanthomonas campestris.
Guar gum
Functions:
-Viscosity binder
-Stabilizer and water binder
Optimum pH range: 4-10
Optimum soluble solids range: 0-80%
Solubility in water: Soluble in both hot and cold water
Gelling conditions: Non-gelling
A linear chain of mannose with single galactose units attached as side chains.
Locust bean gum (LBG)
Meats
-Act as binder
Dairy products (such as ice cream)
-it protects against heat shock and imparts a desirable mouthfeel.
Optimum pH range: 4-10
Optimum soluble solids range: 0-80%
Does not form gel by itself, but can form gel when combined with xanthan gum.
Insoluble in cold water and must be heated to dissolve. The maximum viscosity develop when heated to 95C, then cooled.
Gum arabic
Soft drink emulsion
-As an emulsifier & stabilizer
Beverages such as beer
-promote the stabilization of foam
Volatile flavour compounds
-Act as encapsulation agent to encapsulate the volatile flavour
Confectionery products
-to retard sugar crystallisation and promote emulsification
Least viscous & most soluble
Dissolve easily in hot and cold water
Alginate
Applications
Emulsion like mayonnaise
-stabilizer in emulsion (Propylene glycol alginate)
Beverages
-As thickener & stabilizer
Ice cream
-to avoid crystallisation
Can form gel in cold water with the presence of Ca ion, and the gel is thermo-irreversible.
Made up of blocks of D-mannuronic acid & L-gluronic acid
Carrageenan
Applications:
a) Water dessert gel: Kappa+ Iota
b) Chocolate milk: Kappa, Lambda
c) Canned & processed meats: Kappa
Calcium and potassium ion can form bridges between adjacent double helices through an electrostatic binding to two adjacent sulfate groups, thus stabilizing the network.
Can be divided into:
a) Kappa carrageenan
-Able to form thermoreversible gel
b) Iota carrageenan
-Able to form thermoreversible gel
c) Lambda carrageenan
-Non-gelling
Composed of linear galactan polysaccharides with sulphate content of 15-40%
Pectin
Low methoxy pectin (LMP)
Can be further divided into:
a) Conventional low methoxyl pectin (LMP)
b) Amidated low methoxyl pectin (ALMP)
To form gel:
a) Soluble solid content: 0-80%
b) pH: 2.5-5.5
c) Presence of calcium ion
Heat reversible
DE<50%
High methoxy pectin (HMP)
Applications:
a) Jam with suspended fruits
b) Acid fruits
Can be further divided into:
a) Extra rapid set
b) Rapid set
c) Medium set
d) Slow set
Firm and short structure, clear and transparent, excellent flavour release
To form gel:
a) Soluble solid content: 55-85%
b) pH: 2.8-3.8
Not heat reversible
DE > 50%
Sources of hydrocolloids
Pectin: Peel of citrus fruits or apple pomace
Konjac glucomannan: Armophophallus konjac, K. Koch tuber
Locust bean gum: Seed of carob bean
Carrageenan: Seaweeds
Gum arabic: Acacia senegal L
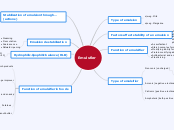
Functions
Secondary Functions
Formation of film
Encapsulation
Control of crystallisation
Suspension of particulates
Stabilisation of emulsion
Primary Function
Gelling agents
Thickening agents
Factors which affect gum properties
Distribution of side chains
Number of side chains
Type of side chains
Monosaccharide composition
Molecular weight