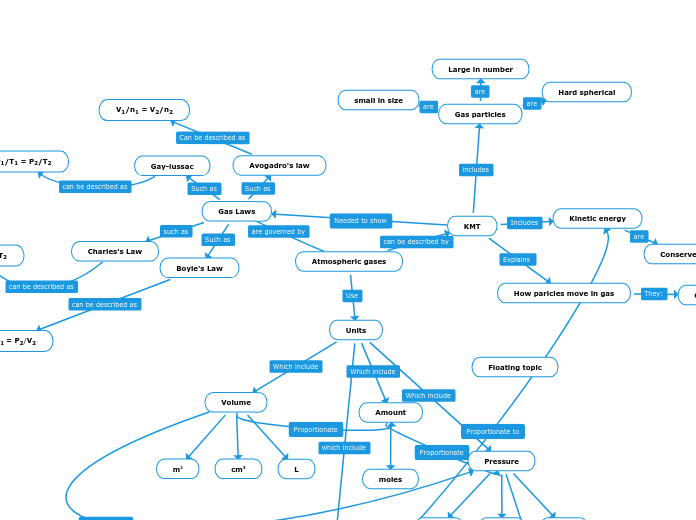
Atmospheric gases
Units
Volume
m3
cm3
L
Temperature
C
K
Pressure
atm
bar
Pa
kPa
Amount
moles
Gas Laws
Boyle's Law
P1/V1 = P2/V2
Charles's Law
V1/T1 = V2/T2
Gay-lussac
P1/T1 = P2/T2
Avogadro's law
V1/n1 = V2/n2
KMT
How paricles move in gas
Continue until collision
Kinetic energy
Conserved
Gas particles
small in size
Large in number
Hard spherical
Ideal Gas law
SATP
Molar gas volume