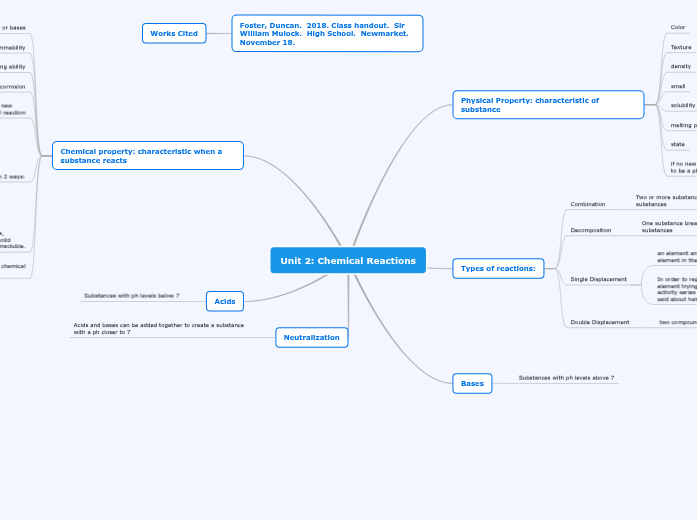
Unit 2: Chemical Reactions
Physical Property: characteristic of substance
Color
Texture
density
smell
solubility
melting point
state
if no new substance is created a reaction is considered
to be a physical reaction
Types of reactions:
Combination
Two or more substances to make a lesser number of substances
Decomposition
One substance breaks apart into a fewer amount of substances
Single Displacement
an element and compound react and the element replaces one element in the compound
In order to replace another element in a compound, the element trying to replace it will have to be higher on the activity series of metals in reactivity. The same thing can be said about halogens replacing halogens
Double Displacement
two compounds react to form two different compounds
Bases
Substances with ph levels above 7
Chemical property: characteristic when a substance reacts
Reaction with acids or bases
flammability
bleaching ability
corrosion
A reaction that results in 1 or more new
substances is considered a chemical reaction
In a chemical reaction atoms are neither created
nor destroyed, just simply re arranged
Reactions can be shown in 2 ways:
Word Equations:
Sodium + chlorine = sodium chloride
Chemical equations:
2K + Cl2 = 2KCl
Chemical equations need to be balances
to have the same number of molecules on
either side of the equal sign
For example K + Cl2 = KCl
becomes 2K + Cl2 = 2KCl
to match the number of chlorine
atoms in the first part of the
equation(there are two because
Chlorine is diatomic and will always
be found in pairs when not part
of a compound).
One way to tell when a chemical reaction occurs,
is when a precipitate forms. A precipitate is a solid
forms in a liquid. This means the substance is insoluble.
When a substance is insoluble, it makes it easier to predict if substances will react.
Spectator Ions: ions that appear on both sides of of a chemical equation but don't actually do anything.
Acids
Substances with ph levels below 7
Neutralization
Acids and bases can be added together to create a substance with a ph closer to 7