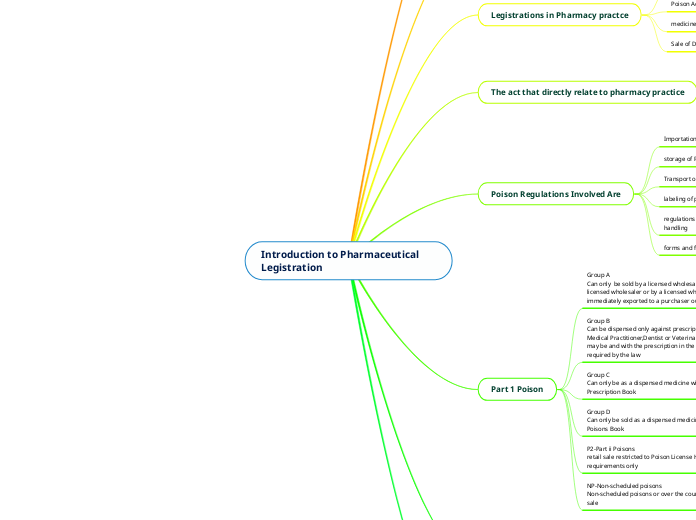
Introduction to Pharmaceutical Legistration
Legistration
Poison Act 1952
sales of Drug Act 1952
Poison Regulations
Control of Drugs & Cosmeyics Regulation 1984
Advertisements Act (Medicines 1956)
Pharmacy Law
Importation,manufacturing,sale and use of poisons and products
practice of pharmacists
advertisements relating to products,medical skills and services
Legistrations in Pharmacy practce
Registration of Pharmacy Act 1951
Poison Act 1952-poison list
medicines (Advertisement and Sales)Act
Sale of Drugs Act 1952
The act that directly relate to pharmacy practice
Poison Act 1952
Poison List
Dangerous Drugs Act 1952
Poison Regulations Involved Are
Importation of Poison
storage of Poison
Transport of poison
labeling of poison
regulations for hospitals & institutions realting of poisons
handling
forms and fees for recording and documentation
Part 1 Poison
Group A
Can only be sold by a licensed wholesaler to a another licensed wholesaler or by a licensed wholesaler to be immediately exported to a purchaser outside Malaysia
Group B
Can be dispensed only against prescription of a Registered Medical Practitioner,Dentist or Veterinary Surgeon as the case may be and with the prescription in the correct form as required by the law
Group C
Can only be as a dispensed medicine with entry in the Prescription Book
Group D
Can only be sold as a dispensed medicine with an entry in the Poisons Book
P2-Part ii Poisons
retail sale restricted to Poison License Holder,Labelling requirements only
NP-Non-scheduled poisons
Non-scheduled poisons or over the counter products for retail sale
Psychotropic Substance can only be supplied or sold by:
A regitered medical practitioner/dentist/veterinary surgeon for the purpose of treatment only
A licensed pharmacist upon a prescription prescribed by medical practitioner/dentist/veterinary surgeon
A pharmacist working in Government hospital/clinic/dispensary
The prescription must have
written,signed and dated by the prescriber itself
full name,address and no tel of the prescriber
Age,full name and address of the patient
total amount of psychotropic substance to be supplied and dose
specify the number of times (not exceeding three) the psychotropic substance may be supplied at what intervals