Alkynes
Alkanes but with triple bond between carbon atoms.
- Unsaturated: Doesn't have max hydrogen atoms per carbon atom
- 1 sigma bond, 2 pi bonds
- Bond Order 3
Ex. C2H2
Alkenes
Alkanes but have double bonds between carbon atoms.
- Unsaturated: Doesn't have max hydrogen atoms per carbon atom
- 1 sigma bond, 1 pi bond
- Bond Order 2
Ex. C2H4
Solutions
Alkanes
Saturated compound with carbon atoms + multiple hydrogen atoms.
- Saturated: Max hydrogen atoms (3) per carbon atom
General formula: CnH2n+2
No double bonds between carbon atoms.
Decane
C10H22
Nonane
C9H20
Octane
C8H18
Heptane
C7H16
Hexane
C6H14
Pentane
C5H12
Butane
C4H10
Propane
C3H8
Ethane
C2H6
Methane
CH4
Concentration
Bascitity
pH >7
- Higher concentration of hydroxide ions in solution
- 10x the concentration of OH- per level of pH
- Dissociates in water to form OH- and M+ ions
*Strength of base refers to its dissociation ability rather than concentration.
Strong Base: MOH --> M + OH
Ex. LiOH --> Li + OH
- All alkali bases are strong
- Dissociates completely
- High conductivity (because of ions)
Weak Base: B + H2O --> BH + OH
Ex. NH3 + H2O --> NH4 + OH
- Doesn't dissociate completely
- Slight conductivity (not as much ions)
Acidity
pH <7
- Higher concentration of hydronium ions in solution
- 10x the concentration of H+ per level of pH
- Ionizes in water to form H+ and non-metal ions
*Strength of acid refers to its ionization ability rather than concentration.
Strong Acids: HA + H2O --> H3O + A-
Ex. HCl + H2O --> H3O + Cl-
- All molecules ionize
- High conductivity (because of ions)
Weak Acid: HA + H2O --> H3O + A-
Ex. HF + H2O --> H3O + F- (only some)
- Only some molecules ionize
- Slight conductivity (less ions)
Moles
% Composition
Hydrate Formula
Molecular Formula
Empirical Formula
Types of Reactions
Double Displacement
Neutralization
Forms a gas
NH4 + OH
Ionic Compound + NH3 + H2O
HCO3
Acid + MCO3 --> Ionic Compound + H2O + CO2
Precipitation Reactions
Combustion
Complete: HC + O2 --> H2O + CO2
- When sufficient oxygen is present
Incomplete: HC + O2 --> C + CO + CO2 + H2O
- When insufficient oxygen is present
Other types: element + O2 --> Element oxide
- Synthesis reactions with oxygen can be classified as combustion if light and heat is produced (exothermic)
Single Displacement
Metal swap H from acid
Metal swap Metal
Halogen swap halogen
Metal swap Water
Synthesis/decomp
Bicarbonate
MHCO3 --> MCO3 + CO2 + H2O
Binary Compound
Element + Element
Bases
MOH + H2O
Acid
Nonmetal Oxide + H2O
Carbonates
MCO3 --> MO + CO2
Chlorates
MClO --> MCl + O2
Nitrates
MNO3 --> MNO2 + O2
Types of Bonds
Covalent/Molecular
Atoms bond through shared electrons. Covalent/molecular refers to the bond within a molecule.
IMFS
- Forces which hold molecules together in a structure
- Is broken when changing states or dissolving
H-Bonds
LD
Dipole-dipole
Nonpolar
0-0.5 EN
- Molecules held together through LD IMFS
- These seperate when boiled, dissolved, or melted
Properties:
- Lowest melting/boiling points
- Dissolves in nonpolar solvents, insoluble in polar solvents
- Weakest bonds
Polar
0.5-1.7 EN
- Anything that's neutral overall but has charge seperation
- Molecules held together through dipole-dipole or H-bond IMFS
- These seperate when dissolved, boiled, or melted
Properties:
- Generally high melting/boiling points
- Stronger bonds than nonpolar molecules
- Soluble in polar solvents, insoluble in nonpolar
Ionic
1.7+ EN
- Has no IMFS
- Held together in crystal structure in an equal ratio indictated by subscripts in formula
Properties:
- Very strong bonds, requires lots of energy to break them apart into different states
- High melting and boiling points
- Generally soluble in water through dissociation
- Cations bond to oxygen while anions bond to hydrogen
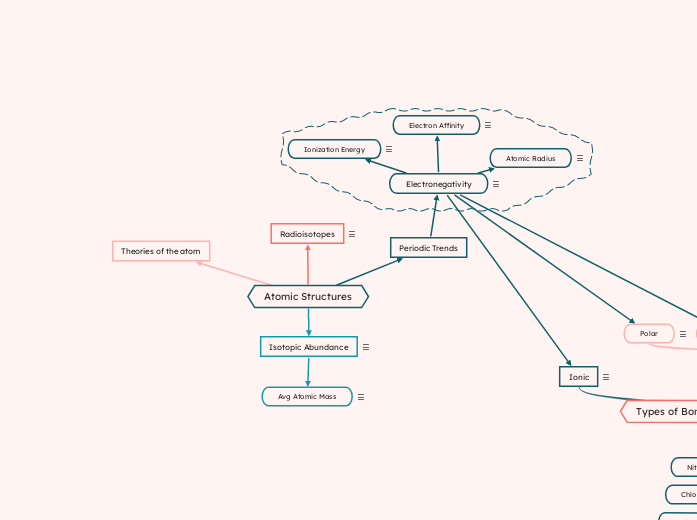
Atomic Structures
Isotopic Abundance
% of a given isotope in nature out of other isotopes of the same element.
Avg Atomic Mass
Avg. mass of all isotopes of a given element.
= (%abundance x amu) + (%abundance x amu)
Radioisotopes
Isotopes that have unstable nuclei and undergo radioactive decay.
Unstable nuclei: When repulsive-electric force of protons is not balanced with the attractive force of neutrons (too many neutrons).
Theories of the atom
Periodic Trends
Electronegativity
The relative ability of an atom to attract electrons in when bonded in a molecule
Electron Affinity
Energy released when electrons are added
Atomic Radius
Distance between nucleus and outermost electrons
Ionization Energy
Energy needed to remove electrons