da Khaliq Eshal mancano 5 anni
373
Compunds
da Khaliq Eshal mancano 5 anni
373

Più simili a questo
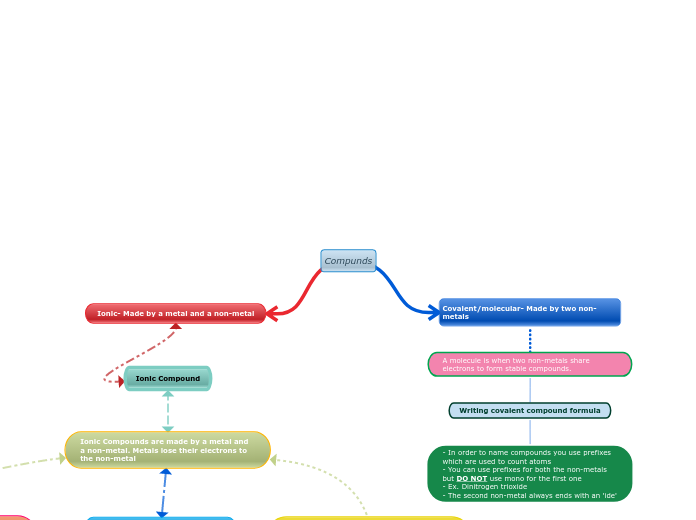
- In order to name compounds you use prefixes which are used to count atoms - You can use prefixes for both the non-metals but DO NOT use mono for the first one - Ex. Dinitrogen trioxide - The second non-metal always ends with an 'ide'