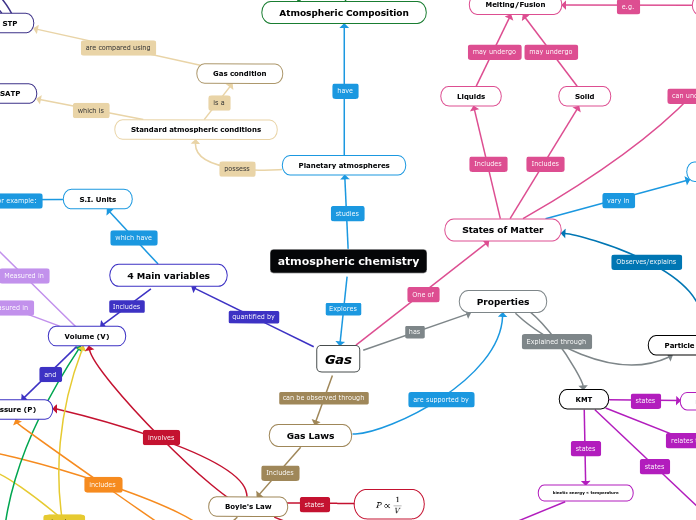
atmospheric chemistry
T = 298.15K
P = 100kPa
T = 273.15K
P =100kPa
SATP
collisions
kinetic energy ∝ temperature
Avogadro's Law
Density
Lots of space between particles
Gas condition
STP
Planetary atmospheres
Atmospheric Composition
Greenhouse Gases
Standard atmospheric conditions
climate change
mol
mole fraction
ratio of moles of one substance vs. total substances in gas mixture
partial pressure
molar mass
g/mol
Molar Constant (R)
Ideal Gas Law
Combined Gas Law
particles are small, spehrical entities
-273.13
Gas is highly condensable, no fixed shape
Charles's Law
Gay-Lussac's law
Boyle's Law
Impacts from particles on surfaces create pressure
particles
Pressure (P)
Temperature (T)
Melting/Fusion
state changes
Liquids
Solid
Particle theory
KMT
States of Matter
Absolute Temperature
number of particles (n)
°C
S.I. Units
K
Pa
L
atmospheric chemistry
Gas
4 Main variables
Volume (V)
Properties
Gas Laws

