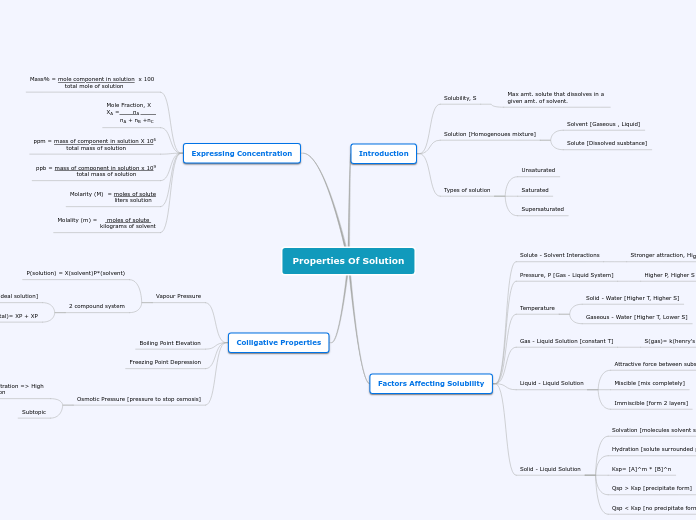
Properties Of Solution
Colligative Properties
Osmotic Pressure [pressure to stop osmosis]
Subtopic
Low solute concentration => High
solute concentration
Freezing Point Depression
Boiling Point Elevation
Vapour Pressure
2 compound system
P(total)= XP + XP
Raoult's Law [ideal solution]
P(solution) = X(solvent)P*(solvent)
Expressing Concentration
Molality (m) = moles of solute
kilograms of solvent
Molarity (M) = moles of solute
liters solution
ppb = mass of component in solution x 109
total mass of solution
ppm = mass of component in solution X 106
total mass of solution
Mole Fraction, X
XA = nA
nA + nB +nC
Mass% = mole component in solution x 100
total mole of solution
Factors Affecting Solubility
Solid - Liquid Solution
Qsp < Ksp [no precipitate form]
Qsp > Ksp [precipitate form]
Ksp= [A]^m * [B]^n
Hydration [solute surrounded by water molecules]
Solvation [molecules solvent surround molecules solute]
Liquid - Liquid Solution
Immiscible [form 2 layers]
Miscible [mix completely]
Attractive force between substances
Gas - Liquid Solution [constant T]
S(gas)= k(henry's law)* P(gas)
Temperature
Gaseous - Water [Higher T, Lower S]
Solid - Water [Higher T, Higher S]
Pressure, P [Gas - Liquid System]
Higher P, Higher S
Solute - Solvent Interactions
Stronger attraction, Higher S
Introduction
Types of solution
Supersaturated
Saturated
Unsaturated
Solution [Homogenoues mixture]
Solute [Dissolved susbtance]
Solvent [Gaseous , Liquid]
Solubility, S
Max amt. solute that dissolves in a
given amt. of solvent.