Biological Molecules
Lipids
phospholipids- major component of cell membranes
steriods
Carbohydrates- fuel and building material
include sugars and polymers of sugars
creating glucose in ring formation as well as linear
Nucleic Acids
mRNA: messenger RNA which controls protein synthesis
Transcription (information in the DNA is used to make mRNA)
Translation (information from the mRNA is used to make proteins)
nitrogenous based used are A, G , C, T
double stranded with complementary base pairing
ribonucleic acid (RNA)
Nitrogenous bases used are A, G ,C, U
polymers made of monomers called nucleotides
5 carbon sugar (pentose), a phosphate group, and a nitrogenous base
purines (A, G)
pyrimidines (C , T) also U in RNA
nucleotide (have a phosphate group)
make a phosphodiester bond
one end has a free phosphate group connected to a 5’ carbon of the sugar, while the other end has an OH group connected to the 3’ end of the end sugar. So we call one end of the nucleic acid 5’ end and the other end is the 3’ end.
nucleoside (do not have a phosphate group
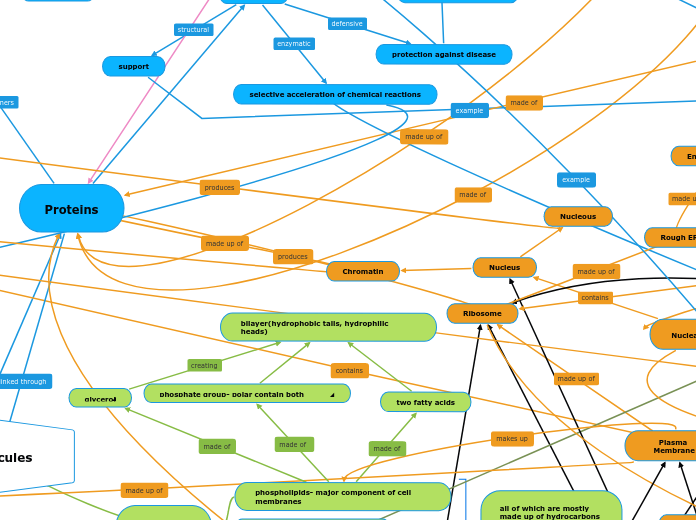
Proteins
selective acceleration of chemical reactions
protection against disease
storage of amino acids
transport of substances
support
response of cell to chemical
stimuli
coordination of an organism’s
activities
movement
amino acids
central (alpha carbon)
main chain
carboxyl group
negative charge
hydrogen
amino group
positive charge
zwitterion in neutral pH within cell
side chain (R group)
hydrophilic
polar
charged
basic
acidic
hydrophobic
nonpolar
peptide bonds
polypeptides
primary
intramolecular polar covalent peptide bond through main chain
secondary
intermolecular hydrogen bond between main chain
alpha helices
beta pleated sheets
tertiary
intermolecular R group interactions cause polypeptide to fold
hydrophobic/van der waals
hydrogen bonds
form intramolecular disulfide bonds through oxidation (only covalent bond between R groups)
ionic bonds
quaternary
2 or more polypeptides come together to form a functional protein
intermolecular R group interactions
dimer
trimer
tetramer
hemoglobin
denaturation-protein unfolds back into primary structure (no longer biologically active)
only peptide bonds remain
renaturation-reverse conditions and test protein function
amino acid sequence determines protein structure
amino acid will go from surface of protein to buried inside
amino acid will go from buried within protein to the surface
Prokaryote
Eukarya
Plantae
Plants, survive by capturing energy from the sun in photosynthesis
Fungi
Animalia
Protista
Bacteria
DNA
Frimbriae
Pili
Nucleoid
Glycocaly
Gas vacuole
Inclusion bodies
Endospore
Peptidoglycan in cell wall
Archaea
Extremophiles
Extreme halophiles
Live in highly saline enviroments
Extreme thermophiles
Thrive in very hot environments
Branching in membrane hydrocarbon tails of phospholipids.
Capsule
Main topic
Main topic
About half the sugar made consumed as fuel for cellular respiration. Sugar is transported to nonphotosynthetic cells as sucrose. Excess sugar is stored as starch in chloroplasts or in the cells of roots, tubers, seeds, and fruits
Endoplasmic Reticulum (ER)
Rough ER
Smooth ER
Metabolic Pathways
Nuclear Envelope
Nucleus
Nucleous
Chromatin
Catabolic Pathways
There is a release energy by breaking down complex molecules into simpler compounds
Anabolic Pathways
There is a consume of energy to build larger, complicated molecules from simpler ones
Subtopic
Polymerization
Energy
Kinetic Energy
Energy associated with motion of molecules or objects
Molecular Motion
Thermal Energy
Movement of Photon
Light Energy
Thermodynamic
System
matter within define region of space
Closed System
Open System
Surrounding
matter in the rest of the universe
Laws of Thermodynamic
First Law
Energy cannot be transferred and transformed, but it cannot be created or destroyed
Second Law
Every energy transfer or transformation increases the entropy of the universe
Gibb's Free Energy
H = G + TS
H = Total Energy (Enthalpy)
G = Gibbs
T = Temperature in Kelvin
S = Entropy
G = DH - TDS
Free-Energy Change (∆G)
∆G = G(final state) - G(initial state)
Exergonic
Energy released, spontaneous
∆G < 0
Energy from catabolism
ATP Cycle
Endergonic
Energy required, nonspontaneous
∆G > 0
Glutamic Acid conversion to Glutamine
∆G for ATP hydrolysis
Energy for Cellular Work
Equilibrium
No net change occurs
∆G = 0
Potential Energy
stores energy
due to position, location, or arrangment
Chemical energy
Molecular Structure
Food
Enzymes
Lowered Activation Energy
Catalytic Cycle
Substrate enter the active site; enzyme changes shape
Optimal Temperature
Optimal pH
Enzyme Inhibitors
Competitive Inhibitors
Mimics the shape of the substrate and competes with the enzyme for the active site. It then blocks the enzyme from sitting on the active site
Noncompetitive inhibitors
Binds to the enzyme away from the active site and alters the shape of the enzyme, so that when the substrate binds to the active site, it won't be as effective.
Allosteric Inhibitor
Inhibitor that enters the additional binding site of the Allosteric enzyme and stablizes the inactive form.
Cooperativity
Binding of one substrate molecule to the active site of one subunit locks all subunits in active conformation.
Feedback Inhibitation
Initial substrate binds to the active site of an enzyme and then the end product goes back and acts as the inhibitor to that same enzyme
Substrate are held in active site by weak interactions
The active site lowers EA and speeds up the reaction
Products are released
Active site is available for two new substrate
Cell signaling
Gap junction
Diffusion of molecules between animal cells
Plasmodesmata
Diffusion of molecules between plant cells
Local signaling
When the releasing cell is in close proximity to the target cell and is able to send the signal molecules through a synapse or extra cellular fluid.
Lind distance signaling
When the signaling cell is far away from the target cell and must use other forms like the blood stream to send the signaling molecule.
Reception
Small and non polar molecules passes through the plasma membrane through a process called simple diffusion
Signal molecule bonds to an internal receptor in the cytoplasm
GPCR will receive a signal and bind/touch a G proteins to remove GDP to activate GTP after activated the G proteins will change its shape and detach from the GPCR and move to its next task
The GPCR will move along the membrane and attach self to an enzyme. Doing so the GPCR will alter the enzyme shape and function the enzyme will then be activated and complete the steps to cellular response.
In this process GTP will use energy to activate the receptor in which able to change back to GDP and will move back to original position. The process can then be repeated from the beginning.
Membrane receptors
Used by non polar signals since they can easily diffuse through the membrane. This receptor is found inside the cell.
G protein linked receptor
Tyrosine kinase receptor
These receptors are used when the molecule is hydrophilic and cannot cross the membrane due to the charge and polarity.
These receptors are made of two polypeptides, each polypeptide has the ability to function as a kinase. As a phosphate groups are added to tyrosines this is referred to as a tyrosine kinase receptor the activated receptor cannot interact with other proteins to bring about a response from the cell
Ion channel receptor
An ion channels receptor will remain close until a signaling molecule binds to it. Once that molecule binds the channel will open.
Once this channel is open specific ions are free to flow into the cell and are able to change the concentration of the cell affecting its function and activity
Once the signaling molecule removes itself from the ion channel, the ion channel closes and ions are no longer free to flow into the cell
Intracellular receptors
Signals that are polar or can not diffuse through the membrane must use this kind of receptors to enter the cell and fulfill its purpose
Transduction
The molecule and receptor bind enters the nucleus and binds to specific gene that controls water and sodium flow
The DNA is converted to RNA because of a gene was turned on
This process used different proteins that are activated throughout the process
Response
The protein a synthesize/proteins synthesis the mRNA is translated into a specific protein which changes the shape and function of the receiving cell
made of glycerol
ester linkage made
fatty acids
no double covalent bonds
unsaturated- commonly found in plant sources and liquid at room temperature
double covalent bonds are present
isomers
cis isomers having hydrogen atoms on the same side of the double bond
trans isomers having hydrogen on opposite sides of the double bond
three fatty acids
dehydration or condensation synthesis
breakdown using hydrolysis
a disaccharide is formed creating a covalent bond called a glyosidic bond/ linkage
storage polysaccharides
glycogen
connected through 1-4 glyosidic linkages and alpha glucose monomers
used in animals
startch
dextran
Structure Polysaccharides
made of beta glucose and connected by glyosidic linkages
help together by hydrogen bonds and form microfibrils
chitin
glycerol^
bilayer(hydrophobic tails, hydrophilic heads)
two fatty acids
phosphate group- polar contain both hydrophobic and hydrophilic parts^
four fused rings
beta isomer the OH group is on top of the structure
used in lplants
Amylose
amylopectin - contains branching
Eukaryote
Animal Cell
Ribosome
Flagella
Centrosome
Centrioles
Cytoskeleton
Microfilaments
Actin
Intermediate Filaments
Keratin
Microtubles
Tubulin Dimer
Microvilli
Peroxisome
Mitochondrion
ATP
Food Vacuole
Extracellular Matrix
Collagen Fibers
Fibronectin
Proteoglycan
Integrin
Cilia
Dyenin
Flagella
Lysosomes
Phagocytosis
Autophagy
Plant Cell
Cell Wall
Secondary Cell Wall
Primary Cell Wall
Middle Lamella
Chloroplast
Plasmodesmata
Vacuole
Central Vacuole
Contractile Vacuoles
Golgi Apparatus
Cell Junctions
Tight Junctions
Desmosomes
Gap Junctions
Cytoplasm
Cytosol
Cytoplasmic Streaming
Vesicle
shape determines function
CELLULAR RESPIRATION
mitochondria
pyruvate oxidation and the citric acid cycle STEP 2
glycolysis STEP 1
electrons are extracted from the glucose and added to an electron carrier NAD+ occurring in the cytoplasm outside the mitochondria
pyruvate formed in glycolysis enters the mitochondria and is oxidized. The product of oxidation enters the citric acid cycle generating more electron carriers, NADH, FADH2
NADH and FADH2 carries electrons down the electron transport chain and generates ATP through oxidative phosphorylation
occurs in the mitochondria the location of the ETC is in the inner mitochondrial membrane
there are 4 complexes in the ETC complexes 1, 3, AND 4 are H + pumps so they're job is to pump H+ against they're concentration gradient they get this energy because when electrons are transferred down energy is released
H+ in the intermembrane space go back down there concentration gradient through a membrane transport protein called ATP synthase the energy associated with the H+ gradient is used to add an inorganic phosphate to ADP to form ATP
1 glucose can form 30-32 molecules of ATP
oxidative phosphorylation STEP 3
catabolic process that uses food sources to form CO2 and water with the release on energy
used to make ATP
aerobic respiration
reduction
oxidation
redox reactions
C6H1206 +6O2 -----> 6CO2 +6H20 + ENERGY
C6H1206 is being oxidized (losing electrons)into 6CO2
These electrons are taken by an electron shuttle using NAD to form NADH+, H+
electrons can be directly transferred to Oxygen but this will cause an explosion due to the release of heat and light energy
an electron transport chain is used that was energy is released throughout each step
6O2 is being reduced (gaining electrons) to become 6CO2
substrate level phosphorylation
an enzyme reacts with a substrate that has a phosphate group
formation of a product and transfer of the phosphate group from the substrate to ADP to form ATP
enzyme hexokinase is used to add a phosphate from ATP to glucose to form gluccose 6P
then converted into fructose 6P
uses enzyme PFK ro convert Fructose 6phosphate to fructose 1,6 biphosphate
6 carbon sugar splits unto 2 molecules of 3 carbons forming DHAP and G3P
G3P id oxidized by transfer of electrons forming NADH a phosphate group is attatched to the oxidized substrate
phosphate group is transferred to ADP and G3P is oxidized to the carboxyl group of an organic acid
enzyme relocates the remaining phosphate group
enolase causes double bond to form in substrate by extracting a water molecule
the phosphate group is transferred from PEP to ADP forming pyruvate
once pyruvate us made if O2 is available it enters the mitochondria and is oxidized to form NADH and acetyl coenzyme A
Acetyl CoA adds its 2 carbon group to oxaloacetate producing citrate
isocitrate us oxidized and NAD+ is reduced
after CO2 is released the resulting 4 carbon molecule is oxidized then made reactive by addition of CaO
alcohol fermentation
pyruvate forms acetaldehyde which is reduced to form ethanol. CO2 is released and in the process of reduction electrons forms NADH are transferred to acetaldehyde recycling NAD+
Lactic acid fermentation
pyruvate is reduced to form lactate and recycling back NAD+ no CO2 is produces
Photosynthesis
leaves
mesophyll cells
choloroplasts
chlorophyll--light harvesting pigments
long hydrocarbon tail inserted in the thylakoid membrane
CH3
CHO
When pigments absorb light, an electron is elevated from a ground state to an unstable, excited state
Electrons fall back down to the ground state, releasing photons that cause an afterglow, giving off light and heat
stomata-microscopic pores for CO2 to enter and O2 to exit
6 CO2 + 6 H2O + Light energy ---> C6H12O6 + 6 O2
6 CO2 + 18 ATP + 12 NADPH + 12 H2O --->
C6H12O6 + 18 ADP + 18 Pi + 12 NADP+ 6 O2 + 6 H2O + 12 H+
light reactions
thylakoid membrane
Non-cyclic (linear) flow of e-
Photosystem I
photon of light absorbed by chlorophyll causes e- to be excited, as they go back to the ground state energy is released
main chlorophyll a molecules (P700) where e- are grabbed by a primary electron acceptor
electrons go to Ferridoxin (Fd) then on to NADP+ to form NADPH
Photosystem II
photon of light is absorbed by chlorophyll fouind in light harvesting complexes
causes e- to jump to excited state, and go back down to ground state, releasing energy
released energy absorbed by another molecule, and process repeats
main reaction center pair of chlorophyll a molecules (P680) where e- are grabbed by an electron acceptor molecule
splitting H2O (O2 is released)
electron transport chain
e- from primary acceptor go down plastoquinone (Pq), cytochrome complex,
Plastocyanin (Pc), ferredoxin (Fd)
chlorophyll molecules of photosystem I
formation of ATP by phosphorylation--energy from ETC used to pump H+ into thylakoid space against concentration gradient, and go back down concentration gradient through ATP synthase
cyclic flow of e-
excess NADPH present
only PSI used--as e- are transferred to Fd, instead of forming NADPH they are recruited to the cytochrome complex and plastocyanin molecules of the ETC
movement of electrons leads to formation of ATP by photophosphorylation
calvin cycle
produces sugar from C O2 with the help of the NADPH and ATP
stroma; outside thylakoid
addition of 3 CO2 from the atmosphere to RuBP using enzyme Rubisco
forms a 6 carbon unstable intermediate which splits to form 6 molecules of 3 phosphoglycerate
6 molecules of ATP and 6 molecules of NADPH used to form 6 molecules of G3P
5 molecules of G3P continue on to make more RuBP and 1 molecule of G3P leaves the cycle to form glucose and other sugars
other processes
C3 plants photorespiration--Rubisco favors to bind O2 instead of CO2, so if CO2 concentration is low, Rubisco will bind whatever O2 is present, releasing CO2; no ATP formed
alternative metods of carbon fixation
if stomata are partly closed, the little CO2 that can enter the leaf is fixed using PEP carboxylase (high affinity for CO2) in Mesophyll cells into a 4 carbon compound
CO2 released into a neighboring sheath cell wherein it is fixed using Rubisco and goes through the Calvin cycle to make sugars
plants open stomata at night and incorporate C O2 into organic acids
Stomata close during the day, and C O2 is released from organic acids and used in the Calvin cycle
Metabolism
DNA
Fredrick Griffith discovered that bacteria are capable of transferring genetic information through transformation
Hershey and Chase discovered that protein was not the genetic material, and that it was DNA
Chargaffs rule: The amount of Adenine equals the amount of Thymine. and the amount of Guanine equals the amount of Cytosine
the structure
nitrogenous base
base pairs are held together by hydrogen bonds
phosohodiester bond
sugar phosphate backbone
replication
the two strands of the double helix must be separated at the ORI (origin of replication) to be able to form a daughter strand. the ORI is a sequence of nucleotides in DNA
the enzume Helicase seperates the two strands to form the replication bubble, SSB (single stranded proteins) makes sure the DNA stays seperated while another enzyme called Topoisomerase helps relieve any strain
Primase makes RNA primers complementary to the DNA parent strand. This causes DNA polymerase lll to add nucleotides only to the 3' end
to connect these nucleotides that are in DNA together we have to use phosphodiester bonds using dehydration/ condensation reactions.
A protein called sliding clamp works with DNA polymerase lll and helps keep it on the parent strand so it does not fall off during replication
along the leading strand the DNA polymerase continuously moves forward towards the replication fork
to form the lagging strand multiple RNA primers are laid down and extended by DNA polymerase lll and making okazaki fragment. the primers are then removed by DNA polymerase l and replaced by DNA nucleotides. an enzyme called ligase seals any gaps by connecting nucleotides with phosphodiester linkages
double stranded with complementary base pairings
the double helix was predicted by the Messleson and Stahl experiment showing us that the helix is semi conservative meaning when the helix replicates each daughter molecule will have an old strand and a newly made strand
in this experiment they found an intermediate band as well as a high density band confirming the double helix was semiconservative
Watson and Crick stated that the specific base pairing suggested a possible copying mechanism for the genetic material where each strand wouls be a parent strand with the information to make another strand
Transcription and RNA Processing
linear flow of information from DNA to protein through the formation of mRNA
a process that forms mRNA from DNA
both transcription and translation occur in the cytoplasm in Prokaryotes, mRNA can be immediately translated
initiation
After RNA polymerase binds to the promoter, the DNA strands unwind, and the
polymerase initiates RNA synthesis
at the start point on the template strand.
elongation
RNA polymerase moves downstream, unwinding the DNA and elongating the RNA
transcript 5' to 3'. In the wake of
transcription, the DNA strands re-form a double helix.
termination
Eventually, the RNA transcript is released, and the polymerase detaches from the DNA.
Translation and protein transport
Initiation
At initiation a small ribosomal subunit binds to am mRNA. The small ribosomal unit then scans the mRNA until it finds AUG, which is the start codon.
An initiator tRNA, that consists of an anticodon of UAC will base-pair with AUG. This initiator tRNA carries the amino acid MET, which is methionine.
An initiator tRNA is made up of a single strand of RNA with about 80 nucleotides. When in three dimension the tRNA is shaped in an upside down L and has the anti codon towards the bottom. On the top side of the upside down L it has a corresponding amino acid
The purpose of the tRNA is to bring the correct amino acid to the mRNA during translation.
A large subunit then joins and becomes a translation initiation complex. Translation factors are also used here to bring all the translation components together.
The initiator tRNA is located in the P site of the large ribosomal subunit, while the A site is open to receive the next tRNA with the corresponding amino acid.
Elongation
After the initiation process comes the elongation step. In the A site of the large ribosomal subunit a new incoming tRNA base-pairs with the mRNA. Many tRNAs will attempt to bind to the codon in the A site but only the appropriate anticodon will bind to the mRNA.
A peptide bond will then form between the amino acid in the P site and the A site. This bond occurs between the amino acid found in the A site and the carbonyl end of the polypeptide in the P site. This bond removes the polypeptide from the tRNA in the P site and adds it to the amino acid in the A site.
The last step in elongation is called Translocation. In this final step the tRNA shift down a site, meaning that the tRNA in the P is shifted to the E site and is released. The tRNA is shifted to the A site is moved the the E site and at the same time the mRNA iOS moved with its corresponding tRNAs. Meaning that the A site will be open for the next appropriate tRNA to bond.
The translocation step will repeat and repeat until it reaches the stop codon, which then leads to the termination process of translation.
Termination
Once the stop codon is reached a release factor will stand in the A site and this will dissociate the complex stopping translation
A stop codon is either UAG, UAA, or UGA. the dissociating of the complex stopping translation is a GTP driven processes as well.
Protein transport- all of protein synthesis begins on free ribosomes. The different sequence of amino acids tell proteins what their final location will be and their function.
During translation an SPR will bind to the peptide stand and will momentarily pause the synthesis. The SPR will then also bond itself to a receptor protein found in the membrane of ER.
This SRP will then leave the signal peptide which causes the polypeptide synthesis to resume its natural course. At the same time we have translocation of the protein in the ER membrane.
The protein is then cut by an enzyme found in the receptor. Once this happens the polypeptide is folded to its final conformation.
The protein the goes through further folding in the Golgi, it is shipped here from the ER through a vesicle.
After this the protein is then released into the cell so it cal reach its designated location. Whether it be another organelle like the nucleus or mitochondria, or secreted from the cell.
transcription occurs in the nucleus forming pre mRNA and translation in the cytoplasm
initiation
A eukaryotic promoter commonly includes a TATA box (a nucleotide sequence containing TATA) about 25 nucleotides upstream from
the transcriptional start point.
Several transcription factors, one recognizing the TATA box, must bind to the DNA before RNA polymerase II can bind in
the correct position and orientation.
Additional transcription factors bind to the DNA along with RNA polymerase II, forming the transcription initiation complex. RNA polymerase II then unwinds the DNA double helix, and RNA synthesis begins at the start point on the template strand.
elongation
RNA polymerase II moves downstream, unwinding the DNA and elongating the RNA
transcript 5' to 3'. In the wake of transcription, the DNA strands re-form a double helix.
termination
sequence AAUAAA is a signal for ribonuclease to make a cut in the newly formed pre mRNA and release it from DNA. At the 5’ end a modified G nucleotide CAP is added, and at the 3’ end a polyA tail is added by polyA polymerase. The 5’cap will be used for translation and the 3’polyA tail helps with stability of the mRNA.
Pre mRNA contains introns and exons. Introns are sequences that need to be removed before translation.
Before the pre mRNA exits the nucleus to be translated it has to remove introns and join together exons. Different genes contain different number of introns.
There is a complex of RNA and proteins called Spliceosome that binds the junctions of the introns and makes cuts to release the introns from the DNA. The exons are then joined together.
Presence of introns help alternate splicing. Different combinations of exons can be generated through removal of different introns to form different mRNAs and hence different proteins.
nucleotide in DNA where transcription starts (first nucleotide or +1) on template strand
Nucleotides in DNA to the right are labeled by positive numbers
To the left of the transcription start site nucleotides are numbered by negative numbers
Gene Regulation
Eukaryotic Cells
Nucleus
Chromosomes
Compacted Chromatid that are made of DNA (genes) and proteins
Chromatid
The fibrous double stranded DNA to which proteins are attached to, once it's compcted it formed Chromosomes
10nm Fiber
DNA winds around histones to form Nucleosomes "beads." They are then strung together like beads on a string by linker DNA
Nucleosomes
The packaged DNA (in the nucleous) that consist of DNA winding around a Histone protein
Histone
Small protein that DNA wraps around
Histone Core
H2A
H2B
H3
H4
H1
It's not part of the Nuclesome, but is involved in forming the next level of packaging
Linker DNA
The DNA connecting each nucleosome
30-nm Fiber
Interactions between Nucleosomes cause the thin tiber to coil or fold into this thicker fiber
300 nm-Fiber
The 30-nm fiber that forms looped domains that attach to proteins
Gene Expression
Gene makes RNA when needed
Differential Gene Expression
Almost all cells in an organism contains the same genes, because they are all the same BUT have differential expression (dependong on what they are and the difference purposes they want to express)
Regulation at Transcription Initiation
A Eukaryotic promoter (commonly includes a TATA box) about 25 Nucleotides upstream from the transcriptional start point
Several Transcription Factors (one that recognizes the TATA box) binds to the DNA before RNA Polymerase II can bind in the correction position
Additional Transcription Factors bind to the DNA along with RNA Polymerase II, forming the transcription initation complex. RNA Polymerase II then unwinds the DNA double helix, and RNA synthesis begins at the start point on the template strand
Transcription Factors
General
It binds to the promoter and regionsn ear the Promter to bring about basal or background level of Transcription
Specific
Binds to distal control elements caleld Enhancers and bring changes in the level of transcription
Activators
Increases levels of transcription
Repressors
Reduces levels of transcription
Control Elements in DNA
Proximal Control Elements
Sequences in DNA that is close to promoter and binds to general transcription facotrs
Distal Control Elements
Sequences in DNA that are upstream or downstram of DNA and binds to specific transcription factors (like Activators or Repressors)
Enhancers
Groupings of distral control elements which are far from the gene they control
Model for the Action of Enhancers and Transcription Activators
Activator proteins bind to distal control elements grouped as an enhancer in the DNA. This enhancer has three binding sites, each called a distal control Element
A DNA-bending protein brings the bound activators closer to the promoter, where General transcription facotrs, mediator proteins, and RNA Polymerase II are nearby.
The activator bind to certain mediator proteins and general transcription factors, helpingthem form an active transcription initation complex on the promoter
Cell Specific Transcription
Combinatorial Control of Gene Expression
In the Liver Cell, Activator proteins are present that bind to enhancer sequences that increases the expression of Albumin Gene. ON THE OTHER HAND, in the Lens cell, activator proteins are present that bind enhancer sequences of the Crystallin Gene and not the Albumin Gene. In this case (in the Lens), only Crystalline gene has high levels of expression while Albumin has basal or background expression.
Prokaryotic Cells
Cytoplasm
Operons
A cluster of functionally realted genes which are involved in the same pathway in the cell consisting of the coordinated control of a single on-off "switch."
Positive Regulation
The Operon gene expression if ON (expression is at a high level when the activator is bound to the operator)
Negative Regulation
When the Repressor protein is bound to the Operator sequence, then the gene expression if OFF
Lac Opern
It's found in E. Coli and is founded by Francois Job and Jacques Monod in 1962. It is an example of both Positive and Negative regulation.
Stuctural Genes
Lac Y
Lac Z
Lac A
Regulatory Gene
Lac I
Regulatory Region
Promoter
Operator
Operon OFF
Lactose absent, Repressor Active
When there is no Lactose, the Operon is OFF, because you need an enzyme. Due to this Transcription, Lac Z, Lac Y, and Lac A is blocked (thus causing the Operon to be OFF).
Lactose present, Glucose present (cAMP level is low): little lac mRNA is synthesized
If there is no Lactose, then Lac Repressor will be bound to the Operator preventing expression of Lac Operon genes.
The presence of Glucose blocks Adenylyl Cyclase preventing the production of CAMP (if there is no cAMP present then the CAP is inactive, so it can't help the RNAP bind to the promoter.
Lactose present, glucose (cAMP level is high), causing an abundant lac mRNA to be synthesized
When lactose is present, the Lac repressor protein binds Lactose, such that it can no longer bind to the operator sequence. RNAP can now bind promoter for transcription of lac operon genes.
The activator protein is present (because an activator protein is required for function) and it's called CAP (Catabolite activator protein). It is activated by cAMP. Adenylyl cyclase forms cAMP from ATP. cAMP binds CAP and CAP helps RNAP to bind promoter to facilitate transcription, so the operon is ON
Lactose present, Repressor inactive
When there is no Glucose and Lactose is present, then the Operon is ON, because an Inducible Operson (as expression of Lac Z, Lac Y, Lac A genes) is induced by the presence of Lactose