How can I demonstrate an understanding of how the major vocabulary are related and or categorized using formats of:
concept mapping
Objective: Build a concept map with all terms found in Chapter 2

1) Mass: A measure of the amount of matter that an object contains
2) Volume: A measure of the space occupied by the object
/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png)
3) Extensive Property: A property that depends on the amount of matter in a sample
*Matter is classified into 4 types of matter: Solid, Liquids and Gas

4) Intensive Property: A property that depends on the type of matter in a sample, not the amount of matter
/examples-of-pure-substances-608350-v3-5b4cfc5646e0fb005b4d9588.png)
5) Substance: Matter that has a uniform and definite composition
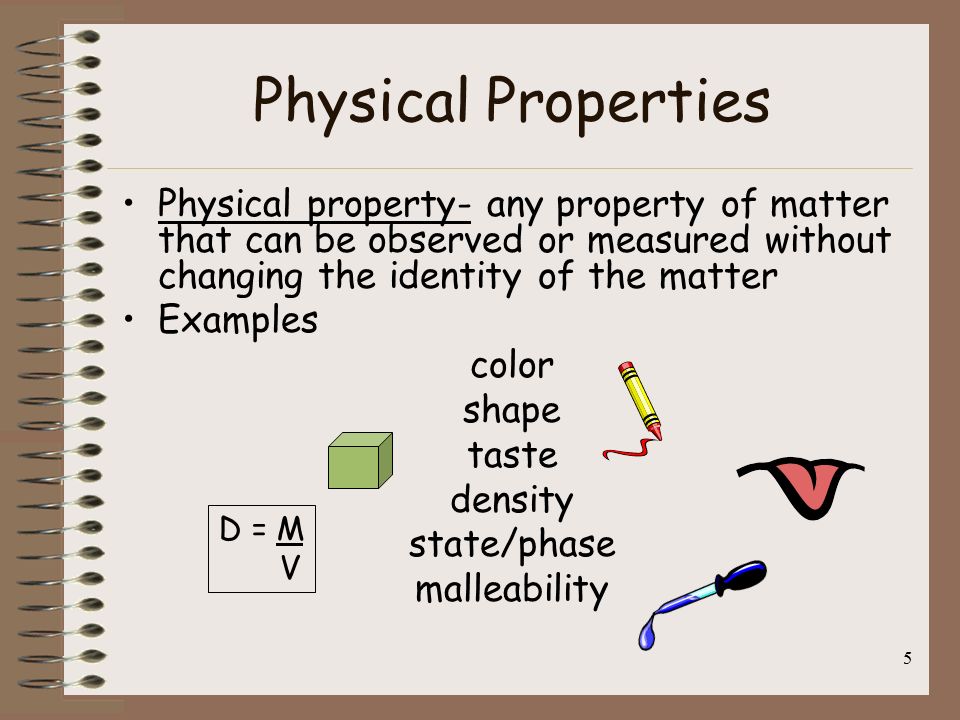
6) Physical Property: A quality of condition of a substance that can be observed or measured without changing the substance's composition
7) Solid: A form of matter that has a definite shape and definite volume; The particles in a solid are packed tightly together
8) Liquid: A form of matter that has an indefinite shape, has a fixed volume and flows; Almost incompressible
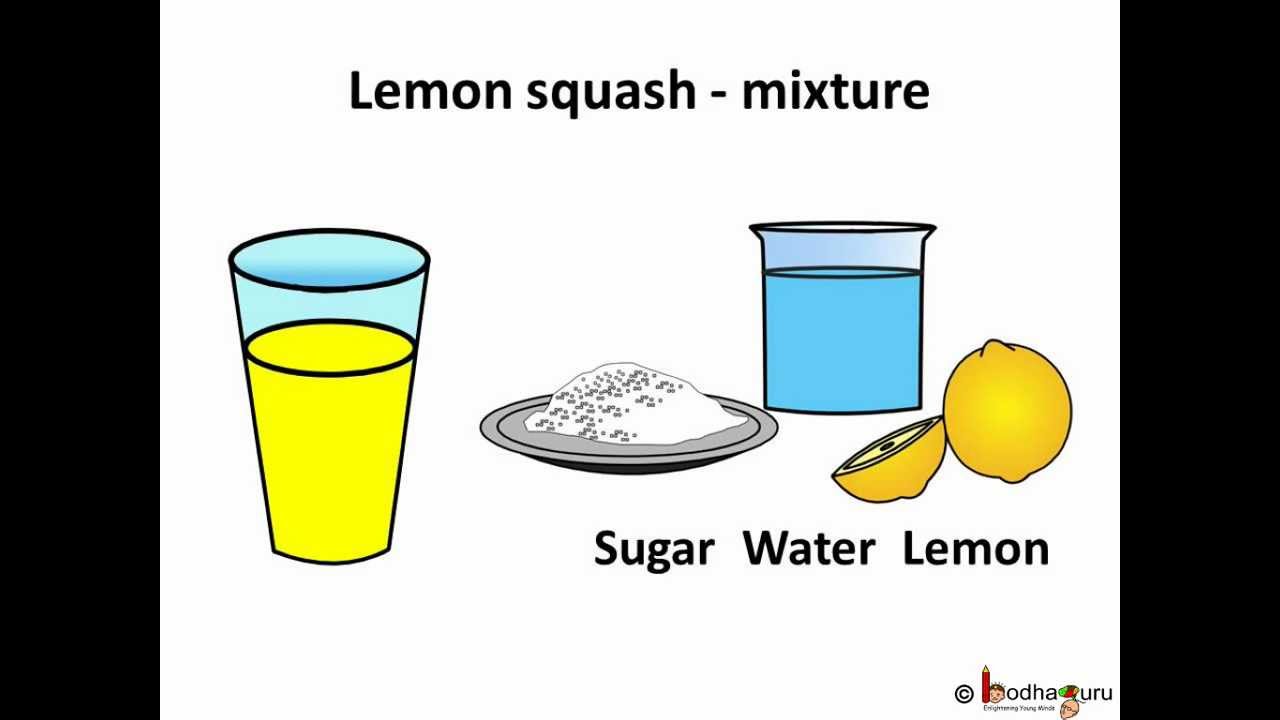
12) Mixtures: A physical blend of two or more components

13) Heterogeneous Mixture: A mixture in which the composition is not uniform throughout; Ex. Chicken Noodle Soup

14) Homogeneous Mixture: A mixture in which the composition is uniform throughout. Ex. Olive oil and vinegar

15) Solution: Another name for a homogeneous mixture

16) Phase: Describing any part of a sample with uniform composition and properties; One single phase is in a homogeneous mixture, while two or more phases contribute to a heterogeneous mixture
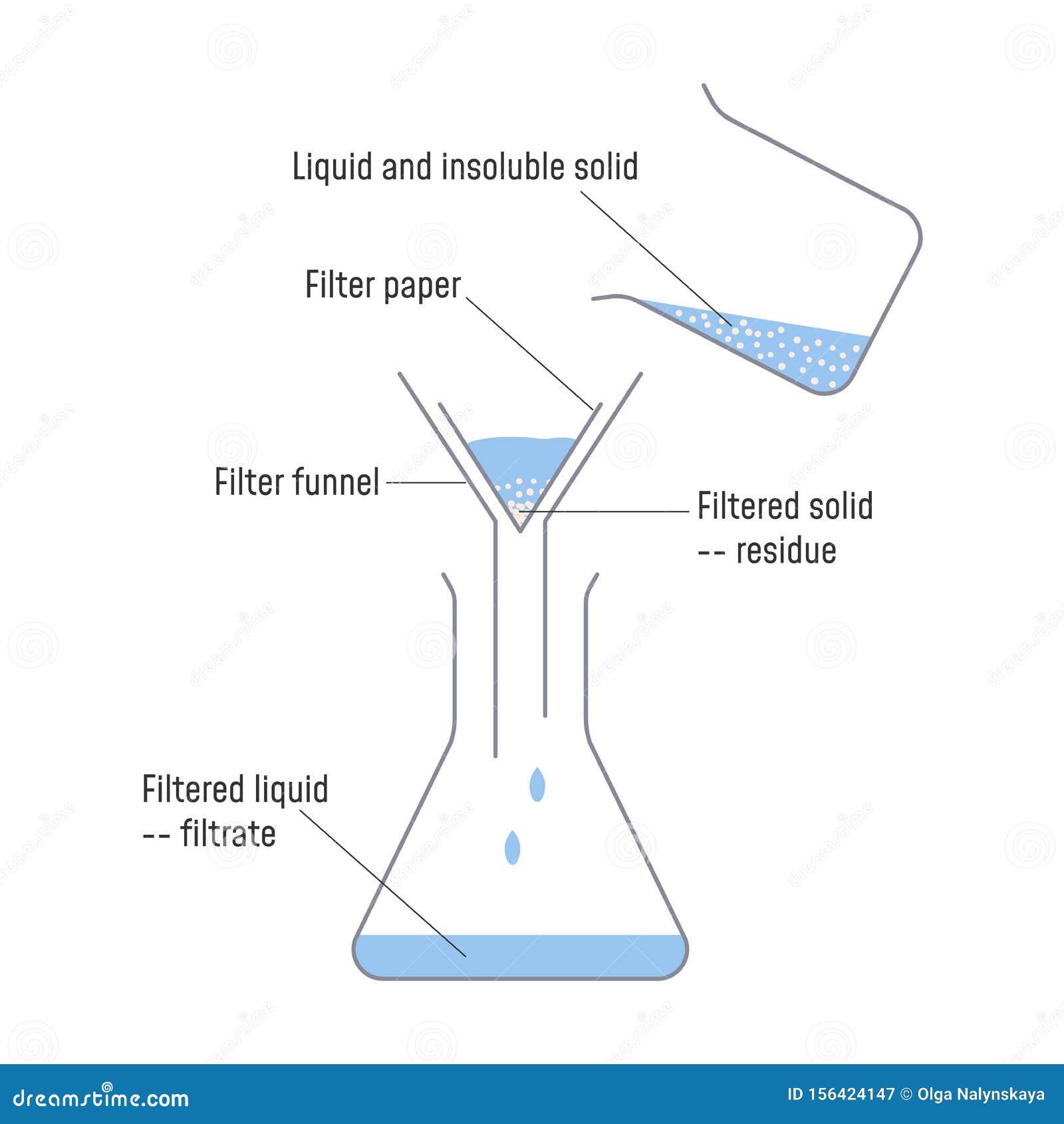
17) Filtration: The process that separates a solid from the liquid in a heterogeneous mixture
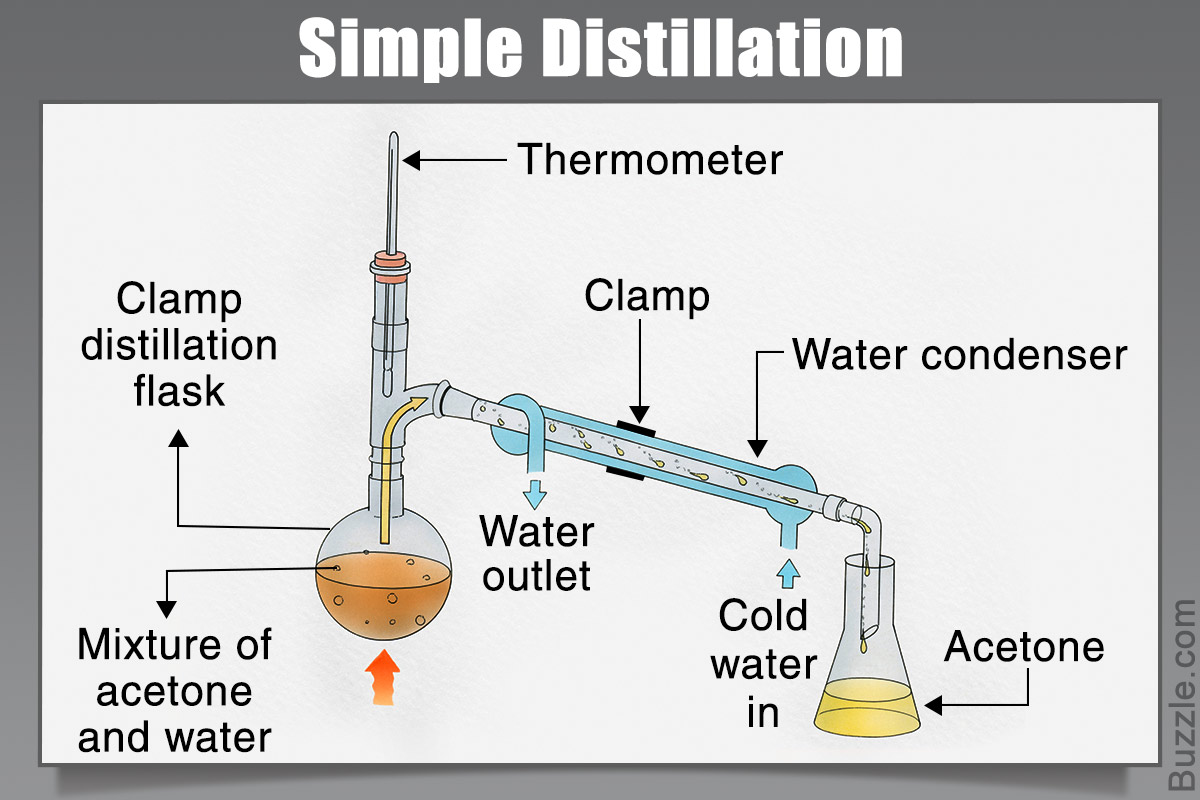
18) Distillation: When a liquid is boiled to produce a vapor that is then condensed into a liquid; One way to separate components
19) Element: The simplest form of matter that has a unique set of properties; Is a substance
20) Compound: A substance that contains two or more elements chemically combined in a fixed proportion
/TC_608334-chemical-change-examples-5aabebea31283400371a753e.png)
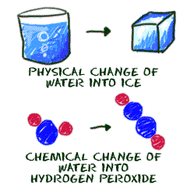
21) Chemical Change: A change that produces matter with a different composition that the original matter; Ex. Heating, Cooling, Etc.
22) Chemical symbol: Represents each element; One or two letter symbol. Ex. Au, Sn
23) Periodic Table: An arrangement of elements in which the elements are separated into groups based on a set of repeating properties.

24) Period: Each horizontal row of the periodic table
25) Group: Each vertical column of the periodic table
26) Chemical Property: The ability of a substance to undergo a specific chemical change

27) Chemical Reaction: Also known as a chemical change; When one or more substances change into a new substance
28) Reactant: When a substance present at the start of the reaction

29) Product: A substance produced in the reaction

30) Precipitate: A solid that forms and settles out of a liquid mixture; A clue that recognizes chemical change

31) Law of Conversation of Mass: The scientific law that reflects these observations is the law of conversation of mass. Matter cannot be neither created or destroyed, only added or taken away.

9) Gas: A form of matter that takes both the shape and volume of its container; The particles in gas are much farther apart than the particles in liquids;
10) Vapor: The gaseous state of a substance that is generally a liquid or solid at room temperature; Ex. Water Vapor