by Faith Stewart 5 years ago
405
Biological Macromolecules back up
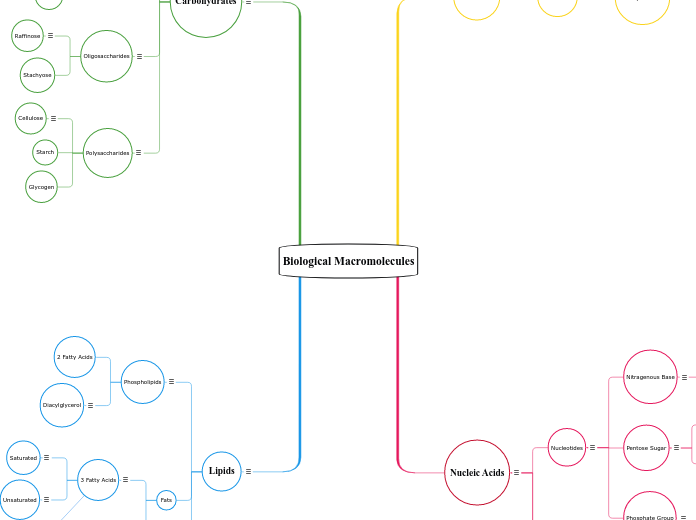
by Faith Stewart 5 years ago
405

More like this
-a nonpolar compound that is made mostly of carbon and hydrogen
-a lipid composed of four carbon rings
-plays an important role in your body
-needs it to make hormones, Vitamin D and help aid in digestion
Hormones
-it has 3 carbon atoms, 8 hydrogen atoms, and 3 oxygen atoms
-the chemical structure of glycerol shows that each carbon atom is bonded to an -OH group
Triglycerol
-large source of energy, but more difficult to utilize than carbohydrates
-3 fatty acids linked to a glycerol molecule
-a hydrocarbon chain with a carboxyl functional group
Unsaturated
-double bonds in the hydrocarbon chains
-are usually liquid and from plant sources
Saturated
-only single bonds in the hydrocarbon chains
-tend to be solid at room temperature and from animal sourcs
-a lipid that contains two fatty acid chains and a phosphate group bound to a glycerol
-polar, hydrophilic head
-nonpolar, hydrophobic tail
-the primary lipid of a cell membrane
-consists of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages
-2 possible forms exist, 1,2-diacylglycerols and 1,3-diacylglycerols
-a molecule that consists of carbon, oxygen, and hydrogen
-a molecule that contains many linked monosaccharides
-large source of energy
-is a molecule consisting of hundreds of carbon, hydrogen and oxygen atoms
-main substance in the walls of plant cells
-important in a humans diet as fibre
-two or more monosaccharides join together by O-glycosidic bonds
-composed of galactose, glucose, and fructose
-can be found in vegetables and whole grains
-two monosaccharides joined by a dehydration synthesis reaction
-a molecule composed of two monosaccharides
-produced natuarlly in plants
-common sugar
-the simplest form of a carbonhydrate
-is a monosaccharide containing six carbon atoms
-found in fruits and plants
-are the assembly instructions for all proteins in living organisms
-sugar-phosphate backbone
-4 bases (G,C,A,U)
-single-stranded
-sugar-phosphate backbone
-5' end has phosphate, 3' end has the sugar
-four bases projecting from the backbone (A,T,C,G)
-double-stranded
-strands run antiparallel to each other
-hydrogrn bonds form between complimentary bases on opposite strands (G forms 3 bonds with C, and A forms 2 bonds with T)
-twists into double-helical formation
-are the buiding blocks of nucleic acids
-are liked together by a sinlge bridging phosphate group between the 5'-carbon of one sugar and the 3' -carbon of the next sugar
-a molecule containing one atom of phosphorus covalently bound to four oxygen residues
-relatively reactive molecules that form phophoester fonds by the interaction with hydroxyl groups
-is a monosaccharide ("simple" sugar)
-has five carbon atoms
Deoxyribose
-found in DNA
-molecules bound to both a phosphate group and either a purine or a pyrimidine
-helps form the backbone of DNA molecules
Ribose
-found in RNA
-is a "normal" sugar, with one oxygen atom attached to each carbon atom
-an organic molecule with a nitrogen atom that has the chemical properties of a base
-bonds nucleic acids together
ACGTU
-the nitrogenous bases in DNA are adenine (A), guanine (G), thymine (T), and cytosine (C)
-the nitrogenous bases in RNA are adenine (A), guanine (G), uracil (U), and cytosine (C)
ATP
-is a molecule that carries energy within the cells
-referred to energy currency of the cell
-a large molecule consisting of many amino acid subunits
-joined by peptide bonds and folded into a specific three-dimensional shape
-is a molecule containing a carboxyl group, an amino group and a side group (R group)
-the difference in R groups is what distinguishes amino acids from each other
-there are 20 different amino acids (meaning 20 different R groups)
-unique linear sequence of amino acids in each polypeptide chain
-20^2= 400 combinations of two amino acids
-20^20 ..... virtually limitless number of combinations
Secondary Structure
-hydrogen bonding between different parts of the amino acid backbone creates two common secondary structures
Beta Sheet
-two or more segements of a polypeptide chain line up next to each other
-forming a sheet-like structure held together by hydrogen bonds
Alpha Helix
-in an a helix, the carbonyl of one amino acid is hydrogen bonded to the amino H (N-H) of an amino acid that has four down the chain
-coiled structure in filamentous and transmembrane proteins
Tertiary Structure
-3 dimensional shape due to intermolecular reactions between R- groups
Quaternary Structure
-many proteins are composed of two or more polypeptides joined together
-held together by the same types of bonds as in the tertiary structure