number of electrons shared
2
4
6
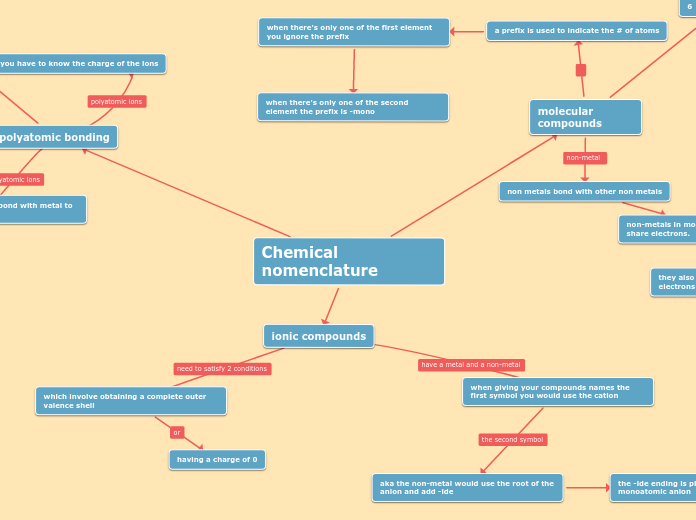
Chemical nomenclature
molecular compounds
a prefix is used to indicate the # of atoms
when there's only one of the first element you ignore the prefix
when there's only one of the second element the prefix is -mono
names of covalent bond
single
double
triple
non metals bond with other non metals
non-metals in molecular compounds have to share electrons.
they also create a covalent bond when electrons are shared
polyatomic bonding
polyatomic anion contain oxygen
when there's 2 oxyanions formed in one element the one with more oxygen the name ends in -ate and the one with less oxygen ends with -ite
also known as a radical bond with metal to form ionic compounds
you have to know the charge of the ions
ionic compounds
when giving your compounds names the first symbol you would use the cation
aka the non-metal would use the root of the anion and add -ide
the -ide ending is placed at the end of a monoatomic anion
which involve obtaining a complete outer valence shell
having a charge of 0