by Adanna Onyeka 3 years ago
353
Final Concept Map
by Adanna Onyeka 3 years ago
353

More like this
transport protein - ATP Synthesis
Citric acid cycle begins
Citrate
Isocitrate
a-ketoglutarate
Succinyl CoA
Succinate
Fulmarate
Malate
Oxaloacetate
Pyruvate
Water/H2O
Protons
Phosphate
Electrons
Single-stranded Binding Protein (SSBs)- enzyme
Primase-enzyme
Lagging strand
Okazaki fragments
DNA ligase- fills in the gaps left by removed RNA primase with nucleotides complimentary to the parent strand
Leading strand
Parental DNA
DNA polymerase III
Daughter strand
5' to 3' direction
Chargaff's rule- states purines and pyrimidine must be paired with one another, specifically the same proportion of adenine to thymine and guanine to cytosine
sliding clamp
Inactive
glucose is present
Active
Lactose present no glucose
Lactose present, CAP is active
Lactose present, cAMP is present
2)Several transcription factors, one recognizing the TATA box, must bind to the DNA before RNA polymerase II can bind in the correct position and orientation.
3)Several transcription factors, one recognizing the TATA box, must bind to the DNA before RNA polymerase II can bind in the correct position and orientation.
Inhibitor: reduces level
Activators:increases level
The 30-nm fiber forms looped domains that attach to proteins
Interactions between nucleosomes cause the thin fiber to coil or fold into this thicker fiber
DNA winds around histones to form nucleosome “beads”
Made of H2A, H2B, H3, H4 at the core
First level of packaging involves attaching proteins (histones) to DNA
Signal-recognition particle carries ribosome to endoplasmic reticulum.
SRP leaves, protein synthesis continues until finished. Then, signal peptide is cleaved and the polypeptide leaves the ribosome and enters the ER.
Protein travels in a vesicle to the Golgi apparatus where it is modified. Modifications include glycosylation, the addition of carbohydrates to proteins to make glycoproteins.
Protein can be carried in vesicles to lysosomes or the plasma membrane to become a membrane protein or be secreted.
Peroxisomes, mitochondria, chloroplasts (in plants), nucleus
Instead, a release factor sits in the A site, disassociating the complex, stopping translation. Disassociation is driven by GTP.
Peptide transferase catalyzes the formation of a peptide bond between the amino group of the new amino acid in the A site and the carboxyl end of the polypeptide in the P site.
Ribosome translocates the tRNA in the A site to the P site. The empty tRNA in the P site is moved to the E site, where it is released. mRNA moves with bound tRNA, bringing the next codon to the A site.
Large ribosomal subunit completes initiation complex. GTP provides energy for assembly. The initiator tRNA is in the P site. The A site is available for the next tRNA.
a 5'cap is added to the pre-mRNA
a 3' poly-A tail is added by polyA polymerase
the pre-mRNA is now ready to undergo processing
RNA Processing
introns are cut out by spliceosome
exons are brought together through spliceosome
mature mRNA is created and ready for translation into proteins
exon-material containing suitable nucleotides for protein function
intron-filler material
RNA polymerase ii unwinds DNA and then adds RNA nucleotides
the RNA being created resembles the non-template strand of DNA 5'-3'
pre-mRNA strand is now created 5'-3'
start point-point of transcription
TATA box-using for recognition of transcription factors
Transcription Factors-used to RNA polymerase ii can bind in the correct position
RNA Polymerase ii-add RNA nucleotides
RNA polymerase ii and Additional transcription factors bind in order to make a transcription initiation complex.
transduction
response
target cell that receives the signal molecule
Intracellular receptors:in cytoplasm & nuclues
steroid hormone aldosterone
Membrane receptors
includes
Ion channel receptor
when a signal molecule binds to the receptor, the gate allows a specific ion like sodium or calcium through the channel in the receptor
movement of ions through these channels may change the voltage across membranes
this would trigger action potential
Tyrosine kinase receptor
Polypeptide on dimerization functions as a kinase
it takes a phosphate group from ATP and adds it to another polypeptide
G protein linked receptor
signal molecule binds to the GPCR
slight alteration in the shape of GCPR allows for the G protein to bind to it
GDP is replaced with GTP on the G protein
G protein with GTP bound to it is active and it can now activate a nearby enzyme
all of the above steps occur in reception
Hormonal Signaling
Synaptic signaling
Paracrine signaling
Oxaloacetate(4C) is formed
malate(4C) is formed
Bundle Sheath cell
Pyruvate (3C)
PEP (3C)
CO2
calvin cycle
sugar
vascular tissue
Phase 1 Carbon Fixation
as CO2 binds with rubisco it creates a short lived intermediate
after the short lived intermediate 3-Phosphoglycerate is made
with the introduction of 6 ATP and excretion of ADP 1,3-Bisphoglycerate is made.
with the introduction of 6 NADPH and the excretion of NADP+P we enter a new phase
Phase 2 Reduction
Glyceraldehyde-3-phosphate (G3P) is created by phase 1 and is the main sugar used by plants
Phase 3 Regeneration of the CO2 acceptor
3 ATP are introduced cause 3 ADP to leave
this creates Ribulose bisphosphate (RuBP)
NADP+P
light and water enter the complex
electrons from water are attached to the chlorophyll as protons and O2 are excecated out. light then causes the electrons to jump to an excited state
as the electrons jump to an excited state they are accepted by the primary acceptor
the electrons then go out a electron transport chain causing ATP to be released. the chain consist of plastoquinone (Pq), Cytochrome Complex, and Plastocyanin (Pc)
Photophosphorylation: ATP from ETC is used to pump H+ into thylakoid space. H+ diffuses down its concentration gradient through ATP synthase, forming more ATP.
Photosystem I(P700)
Cyclic Electron Flow-only used when the cell needs more ATP
Cytochrome Complex to the Plastocyanin creating ATP
plastocyanin then brings its energy to the chlorophyll
electrons get excited to the primary acceptor
electrons are then taken by Ferredoxin back to the Cytochrome Complex
as the electrons for the ETC enter the chlorophyll and more light exciting them they enter the primary acceptor
this causes another ETC consisting of ferredoxin (Fd) to NADP+ reductase.
NADP(+ )+ 2 H+ binds with NADP+ causing NADPH+H+ to be formed
Cellular response is activated after the transduction pathway is completed.
Expression of a gene
Phosphorylation cascade
Activation of relay molecule (small, water-soluble molecule/ion), triggered by reception of ligand
Activation of a protein kinase 1
Activation of protein kinase 2 as protein kinase 1 transfers a phosphate group to it
Activation of an inactive protein as protein kinase 2 transfers a phosphate group to it
Activated protein triggers cellular response
Ligand (signaling molecule) binds to membrane receptor (e.g., GPCR)
GCPR adds GTP to G protein, which then activates membrane enzyme
Common relay molecule: Cyclic AMP
AMP after it activates the next step. Converted by phosphodiesterase (PDE).
ATP using the enzyme Adenylyl cyclase
Prokaryotes - A type of organism that is single celled, lacks a nucleus, and contains no membrane bound organelles.
This kind of organism utilizes aerobic respiration when oxygen is present, but can also use fermentation when oxygen is not.
Organisms that cannot tolerate environments with oxygen present as it would lead to cell death and instead utilize fermentation anaerobic respiration.
Organisms that require oxygen for cell respiration
Prokaryotic cells don't have a membrane bound organelle for their DNA, rather they have a recognized region where DNA is located known as the nucleoid.
Materials Present
Controls the synthesis of proteins and provides essential messenger functions for DNA.
Its structure varies from DNA in that it has ribose sugars instead of deoxyribose, is single-stranded, and has uracil instead of thymine
Bacterial Genome
Endospores are essential for bacteria living under extreme conditions. It protects the DNA of the cell by making a dormant and resistant cell that can survive various circumstances.
As the rest of the cell disintegrates, the DNA is protected by the multiple layers of cell wall material surrounding it, forming the endospore.
Gas vacuoles assist in buoyancy for flotation in aquatic environments.
This structure surrounds the cell wall of bacteria as a sticky later of polysaccharide or protein, and helps the cell for adhesion and protection.
The flagella is important for the cell's motility, and contains a hook a basal body. It can exist either around the surface of the cell, or be concentrated in one area.
Ribosomes are present in both prokaryotes and eukaryotes, and are essential for protein synthesis.
The cytoplasm consists of gel-like cytosol, water based solution with ions and small molecules, and makes up the internal part of the cell inside the plasma membrane.
Present in all prokaryotes, the cell wall maintains the cell's shape, and provides protection and support. This structure is made of modified sugars with short polypeptides called peptidoglycan.
Peptidoglycan
This substance that makes up the cell wall is unique to bacteria, and can be used to target them specifically.
Fimbriae are short abundant structures used by bacteria to stick to other bacteria, while longer pili assist in transferring DNA between cells.
The plasma membrane is a selectively permeable barrier for the cell. It provides boundaries between the inside of the cell and its environment, facilitates the transport of nutrients and waste, and is where metabolic processes occur.
Factors of fluidity
The weak Van der Waal forces present in the membrane allow for the movement of lipids. The fluidity of the membrane however is determined by a few factors.
Cholesterol embedded within the plasma membrane allows for the regulation of the movement in phospholipids by reducing movement at moderate temperatures and preventing the overpacking of saturated fatty acids at low temperatures by acting as a buffer.
Types of fatty acids present
The ratio of unsaturated fatty acids to saturated fatty acids also affects the fluidity of the membrane. Saturated fatty acids lack the structural kink that is present in unsaturated fats, allowing the phospholipids to pack together more tightly, and resulting in a more rigid structure.
Temperature
At a specific lower temperature, the membrane's phospholipids change from their more liquid, crystalline phase, to a more gel-like phase.
Transport of molecules
The membrane being selectively permeable means only certain molecules can pass through without assistance.
Molecules that can transport across the membrane
Molecules that cannot
Specific structures
The plasma membrane is made up of an amphipathic phospholipid bilayer, with hydrophilic heads bordering the outside and inside of the cell, and the hydrophobic tails making up the interior of the membrane.
This arrangement is held together by weak Van der Waal forces that allow lipids to move within the membrane and provides fluidity.
The membrane also consists of proteins located on the surface (surface proteins), within the membrane (integral proteins), and with chains of polypeptides (glycoproteins).
A category of prokaryotic micro-organisms
Methanogens
Another prokaryote in the archaea domain are methanogens. These can be found in hydrothermal vents, the guts of animals, and wetlands. They are anaerobic, meaning oxygen is poisonous to them, and remove excess hydrogen.
Extreme thermophiles
This kind of prokaryote can thrive in high temperatures that few other organisms can survive in. They are commonly found in many extreme temperature environments such as hot springs and hydrothermal vents.
Extreme halophiles
These kinds of archaea are able to thrive in extremely saline environments, referred to as extreme as very little amounts of organisms can survive in similar conditions.
Chemoheterotroph
Chemoheterotrophs utilize carbon from organic compounds, and are characteristics of many prokaryotes.
Photoheterotroph
The prefix photo refers to light, which this mode utilizes for energy. This trait is unique to certain aquatic and salt thriving prokaryotes. Since its a heterotroph it utilizes carbon from organic compounds.
Chemoautoroph
Inorganic chemicals
This mode, as an autotroph, means a prokaryote gets its energy from inorganic chemicals.
Photoautorophs
Light
Organisms that utilize light to synthesize organic molecules are known as photoautotrophs, such as cyanobacteria.
proteins
transport, enzymatic activity, cell-cell recognition, signal transduction, intercellular joining, and attachment to the cytoskeleton and extracellular matrix (ECM)
Transmembrane proteins have an extracellular and cytoplasmic side
Ion channels
sodium potassium-pump, potassium channel, sodium channel
Gated (stretch-gated, ligand-gated, voltage-gated)
Un-gated (always open)
phospholipid bilayer
hydrophobic tail (fatty acid chain) and hydrophilic head (polar) which help control membrane fluidity
selectively permeable
separates organism from the environment
larger molecules
exocytosis
endocytosis
receptor-mediated
phagocytosis
pinocytosis
smaller molecules
osmosis/diffusion
Hypertonic solution
plasmolyzed (plant loses water)
Hypotonic solution
turgid(normal in a plant cell)
Isotonic
active transport
ex:sodium-potassium pump
low->high concentration
protein pumps
facilitated diffusion
channel proteins are needed
passive transport facilitated by proteins
Quaternary: 2 or more polypeptides form a functional protein through R group interactions
Tetramer: 4 PPs
Trimer: 3 PPs
Dimer: 2 PPs
Monomer: 1 PP
Tertiary: R groups interact to from 3D shape
Disulfide bond
Ionic bonds
Hydrophobic interactions
Hydrogen bonds
Secondary: Main chains form hydrogen bonds
Beta-pleated sheet
Alpha helix
Primary: Amino acids connected through peptide bonds
Side chain: R group
Basic: Complete positive charge
Acidic: Complete negative charge
Nonpolar: Has H, CH, or carbon ring
Polar: Has OH, SH, or NH groups
Main chain: Amino & carboxyl groups
Form bilayer in water
Hydrophilic head: Phosphate group
Hydrophobic tail: Glycerol & 2 Fatty acids
Testosterone
Cholesterol
LDL: Carries excess to blood vessels (bad)
HDL: Carries excess to liver for excretion (good)
4 fused rings
Energy storage
3 Fatty acids
Unsaturated
Trans: H on different sides of double-bonded carbons
Cis: H on same side of double-bonded carbons. Has a kink
Liquid at room temp
One or more double bonds & H atoms are notat every position
Saturated
Solid at room temp
No double bonds & H atoms at every position
Glycerol
nitrogenous base
Cytosine (C) & Uracil (U)
ribose sugar
nitrogenous base
Pyrimidines
Cytosine (C) & Thymine (T)
Purines
Adenine (A) & Guanine (G)
phosphate
deoxyribose sugar
Structure polysaccharides
Chitin
Cellulose
Beta glucose & 1-4 glycosidic linkages (no branching)
Storage polysaccharides
Dextran
Starch
Amylase
Alpha glucose & 1-4 and 1-6 glycosidic linkages (some branching)
Amylose
Alpha glucose & 1-4 glycosidic linkages (no branching)
Glycogen
Alpha glucose & 1-4 and 1-6 glycosidic linkages (lots of branching)
Disaccharides
Maltose
Lactose
Sucrose
Monosaccharides
Hexoses: 6-carbon
Fructose
Galactose
Glucose
Beta: OH is above the ring
Alpha: OH is below the ring
Pentoses: 5-carbon
Deoxyribose
Ribose
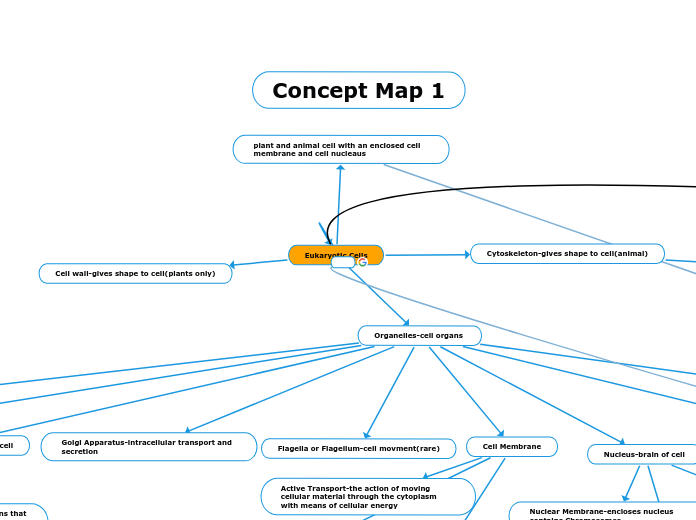
Centriole(only active during cell division in animal cells)
Microtubules-made up of a-tubulin and b-tubulin. also takes part in cell growth and intracellular movement
Osmosis-movement from a high concentration to a power concentration. only solvent molecules move freely
Diffusion-movement from a high concentration to a power concentration. both solute and solvent molecules move freely
protein
Cytoplasm-jelly like substance filling cell
lipids-found in Golgi Apparatus and Cytoplasm
carbohydrates-found in Golgi Apparatus and Cytoplasm
Cytosol-a component of the cytoplasm where organelles and particles are suspended
Vacuoles-space in Cytoplasm
Protein Transport
Gated Channel Protein-a gate must open for a molecule to pass through
Carrier Protein-a protein which has a substance it transports across the cell
Channel Protein-water and small ions pass through
Active Transport-the action of moving cellular material through the cytoplasm with means of cellular energy
Chlorophyll-cellular solar panels
Cellular Respiration-chemical reactions that cause glucose to breakdown into ATP causing cell energy production
ATP-energy in the cell
lipids proteins and one enzyme make up Lysosomes
ribosomes leaving in vesicles from ER to cause protein synthesis and combination with products like carbohydrates and lipids
Smooth ER-ribosomes cannot attach
Rough ER-ribosomes attach
Nucleolus-a structure surrounding the nucleus during interphase
Ribosomes-made up of RNA, it is responsible for protein synthesis
Proteins-make up Ribosomes
Nuclear Membrane-encloses nucleus contains Chromosomes
Chromosomes-holder of genetic information
Chromatin-what makes up Chromosomes
DNA-genetic information