by Ana Lucía Cedeño Tejeida 4 years ago
392
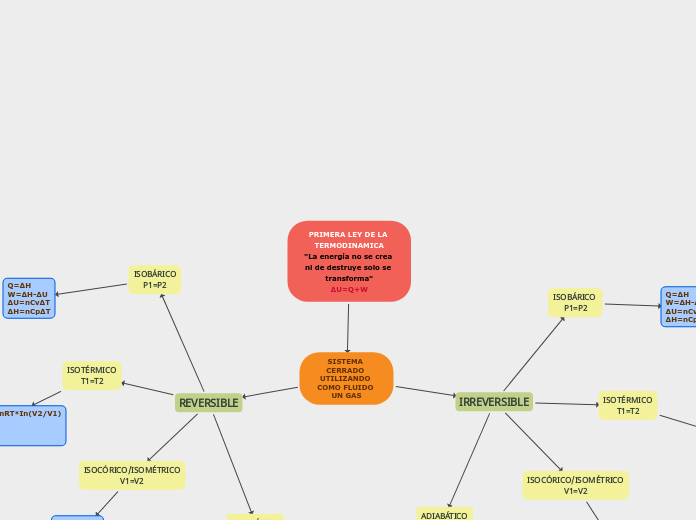
PRIMERA LEY DE LA TERMODINAMICA "La energía no se crea ni de destruye solo se transforma" ΔU=Q+W
by Ana Lucía Cedeño Tejeida 4 years ago
392

More like this
Begin by entering your organisation's name in the central topic, then press Enter.
The four perspectives of the Balanced Scorecard technique help ensure that you are making measurements across all the important areas of your business.
Use the sections below to help identify further measurements, and to group any measurements that have already been identified by examining your stakeholder needs and strategy.
Q=nRT*In(P1/P2)=nRT*In(V2/V1) W=Q ∆U=0 ∆H=0
Q=0 W=-∆U ∆U=nCv∆T ∆H=nCp∆T V1[T1^(Cv/R)]=V2[T2^(Cv/R)] P1[(V1)^k]=P2[(V2)^k]
Q=∆H W=∆H-∆U ∆U=nCv∆T ∆H=nCp∆T
Q=∆U W=0 ∆U=nCv∆T ∆H=nCp∆T
Q=P(V2-V1) W=Q ∆U=0 ∆H=0
Q=0 W=-∆U ∆U=nCv∆T ∆H=nCp∆T [(V2)^(R/Cv)]/V1=T2/T1 P1[(V1)^K]=P2[(V2)^k]