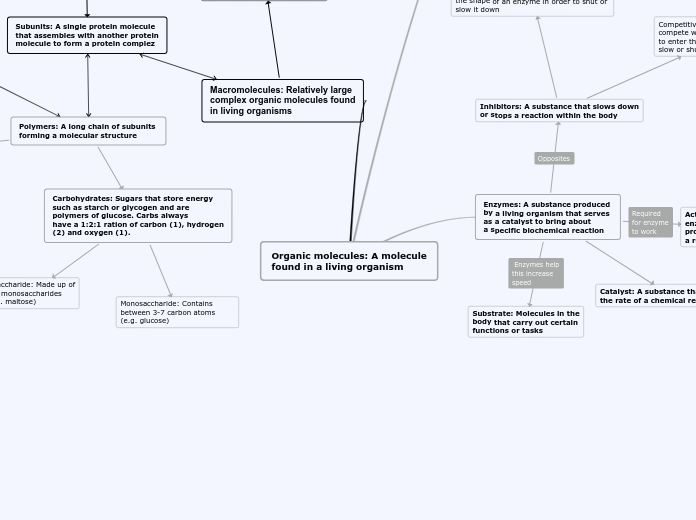
Organic molecules: A molecule
found in a living organism
Main topic
Enzymes: A substance produced
by a living organism that serves
as a catalyst to bring about
a specific biochemical reaction
Catalyst: A substance that increases
the rate of a chemical reactions
Substrate: Molecules in the
body that carry out certain
functions or tasks
Active site: A region on an
enzyme that binds to a
protein / substrate during
a reaction
Induced fit: When the active site
changes shape in order to fit the
reacting substrate
Inhibitors: A substance that slows down
or stops a reaction within the body
Non-competitive inhibitors: An inhibitor
that attaches to a site and forcibly changes
the shape of an enzyme in order to shut or
slow it down
Competitive inhibitors: Inhibitors that
compete with the substrate trying
to enter the active site in order to
slow or shut down a reaction
Macromolecules: Relatively large
complex organic molecules found
in living organisms
Isomer: One of two or more
compounds made of the same
formula but with a different
arrangement of atoms in the
molecule
Monomers: Single subunits (molecules)
that can be attached to make polymers
Hydrolysis: When water
is added to a large molecule
in order to break it down
Subunits: A single protein molecule
that assembles with another protein
molecule to form a protein complez
Lipids: Consists of glycerol paired
with fatty acids used for energy
storage in cell membranes
Saturated fats: No double
covalent bonds between
carbon atoms, therefore
contain all possible H atoms.
They are solid at room temp
and come from an animal source.
Unsaturated fats: Have some
double bonds between carbons
leaving room for additional
H atoms. They are liquid at room
temp and have a plant source.
A
Polymers: A long chain of subunits
forming a molecular structure
Carbohydrates: Sugars that store energy
such as starch or glycogen and are
polymers of glucose. Carbs always
have a 1:2:1 ration of carbon (1), hydrogen
(2) and oxygen (1).
Monosaccharide: Contains between 3-7 carbon atoms (e.g. glucose)
Disaccharide: Made up of
two monosaccharides
(e.g. maltose)
Nucleic acids: Nucleotide chains
that transfer and express
genetic information, they are
polymers of nucleotides
DNA: Self replicating material
present in nearly all living
organisms
RNA: A nucleic acids
present in all living
cells that acts as a
messenger carrying
DNA instructions.
Proteins: Amino acid chains
with a variety of functions
such as transporting and
are polymers of amino acids
Amino acid: Has a central
atom bonded to a hydrogen
atom and three other groups
attached.
Peptide bond: Links
amino acids together
to form peptides and
polypeptides.