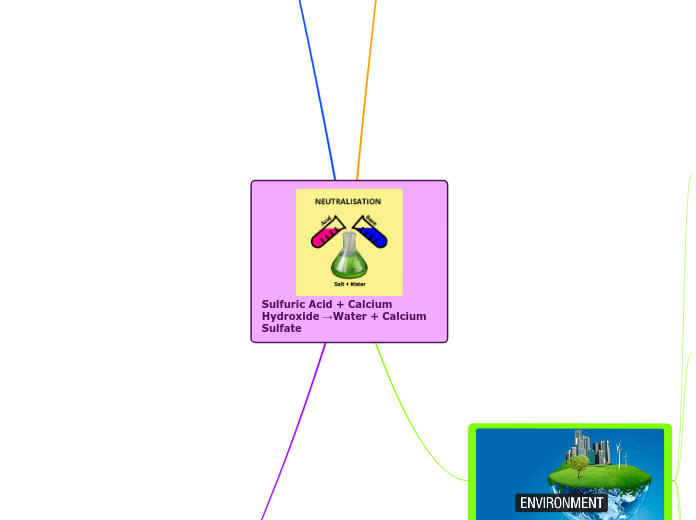
Sulfuric Acid + Calcium Hydroxide →Water + Calcium Sulfate

Word Equation
Sulfuric Acid + Calcium Hydroxide →Water + Calcium Sulfate
Skeletal Equation
H2SO4(aq) + Ca(OH)2(s) → H2O(l) + CaSO4(s)
Balanced Equation
H2SO4(aq) + Ca(OH)2(s) → 2H2O(l) + CaSO4(s)
Chemical reaction
What does it mean?
:max_bytes(150000):strip_icc()/123535121-58b5b3173df78cdcd8ac81de.jpg)
It is a process in which one or more substances, the reactants, are converted to one or more different substances, the products.
There are many types of chemical reactions like synthesis, single displacement, double displacement, etc...
What type?
Neutralization
What is Neutralization?
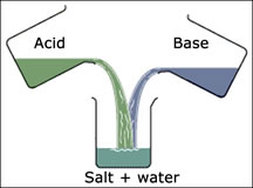
It is a chemical reaction in which an acid and a base react quantitatively with each other to produce a salt. It is also known as a double displacement reaction.
Real life Examples:
Having an acidic stomach due to spicy food can be relieved by taking an antacid (alkaline/ basic).
Depending on the plants you choose to grow, there are ways to treat and neutralize soil that is too acidic or too basic so that the plants will grow happy and healthy.
When plants take nutrients from the soil into their roots they are affected by the pH content of the surrounding soil particles (neutralization takes place.)
The purpose of this reaction:
:max_bytes(150000):strip_icc()/GettyImages-sb10067655by-001-58ca04253df78c3c4feffeed.jpg)
The purpose of this reaction is to produce a salt.
A salt is formed when a cation (positive ion) of a base forms a compound with the anion (negative ion) of an acid.
Reactants?
Calcium Hydroxide [Ca(OH)2]
Properties?

A Colorless crystals or a white powder with a density of 2.21 g/cm³ and melting point of 580 °C

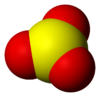
Sulfuric Acid (H2SO4)
Properties?

A colorless or slightly yellow viscous liquid with a pungent odor. It has a density of 1.84 g/mL.
Products?

Calcium Sulfate (CaSO4)
Properties?

An Odorless, white amorphous or crystalline solid or powder.
Water (H2O)
Properties?

Water is colorless and tasteless liquid. It has a high melting and boiling points.
Energy requirements of Neutralization

This reaction is an exothermic reaction, meaning that it produces heat due to the acid and base being neutralized. The enthalpy of neutralization is negative.

Heat produced?
H = E + PV is the formula to find how much heat is produced. First step is to find how many moles there are in the equation.

Are there other products that are not useful in this reaction that are considered to be wastes?
No, all the products are useful in this reaction because calcium sulfate is the reason Gypsum and Plaster of Paris are created. The other product which is water is not wasteful. The reactants are what create calcium sulfate, which is why they are not a waste as well.
Where do they get the reactants from?
Sulfuric Acid
Steps involved in the manufacturing of sulfuric acid (Contact Process)
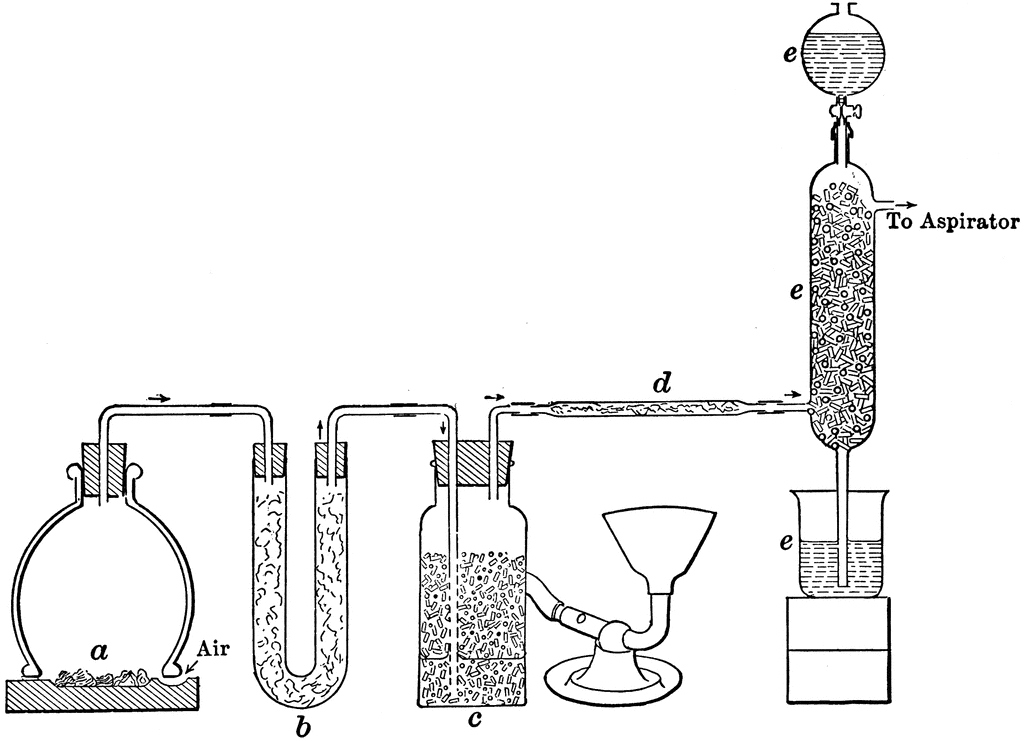
1. Preparation of sulfur dioxide.
2. Conversion of sulfur dioxide into sulfur trioxide.
3. Conversion of sulfur trioxide formed into concentrated H2SO4.
Calcium Hydroxide
Calcium hydroxide is produced commercially by treating lime with water: CaO + H2O → Ca(OH). In the laboratory it can be prepared by mixing aqueous solutions of calcium chloride and sodium hydroxide.
Are there any environmental impacts in the way they obtain the reactants?

Sulfuric Acid

When sulfur dioxide combines with water and air, it forms sulfuric acid, which is the main component of acid rain. Acid rain can: cause deforestation and acidify waterways to the detriment of aquatic life.

To prepare sulfur dioxide we need to burn sulfur, creating air pollution.

Calcium Hydroxide

Calcium hydroxide absorbs carbon dioxide readily from the air, changing to calcium carbonate (CaCO3). For this reason, the compound often is contaminated with the carbonate unless it is kept in tightly sealed containers.
Subtopic

Final Product
Calcium Sulfate
What is it?

It is a naturally occurring calcium salt.

Types of Technology used in this Reaction?

Gypsum.
What is it?

Calcium Sulfate commonly known in its dihydrate form, CaSO4∙2H2O, a white or colourless powder called gypsum
How Does This technology Make the Reaction More Efficient (run better)?

Gypsum helps the environment and it is useful, without it soil conditioner wouldn't exist. The soil amendment that improves the soil structure by increasing aeration, water holding capacity, and nutrients.

It is also the reason why Plaster of Paris is created and that technology is very useful as well.
Used as?

As uncalcined gypsum, the sulfate is employed as a soil conditioner.

Calcined gypsum is used in making tile, wallboard, lath, and various plasters.

When gypsum is heated to about 120 °C (250 °F), it loses three-quarters of its water, becoming the hemihydrate CaSO4∙1/2H2O, Plaster of Paris.

Plaster of Paris
What is it?
How is it Created?
Plaster of Paris is prepared by heating calcium sulfate dihydrate, or gypsum, to 120–180 °C (248–356 °F)

It is a quick-setting gypsum plaster consisting of a fine white powder (calcium sulfate hemihydrate), which hardens when moistened and dry.
Advantage?

Plaster of Paris does not generally shrink or crack when dry, making it an excellent medium for casting molds.
How Does This technology Make the Reaction More Efficient (run better)?
Plaster of Paris is used to help and benefit people in many ways like the examples listed below. Plaster of Paris makes the reaction more efficient as it creates many useful things in life that people desire or need.
What is it used for?

It is commonly used to precast and hold parts of ornamental plasterwork placed on ceilings and cornices.
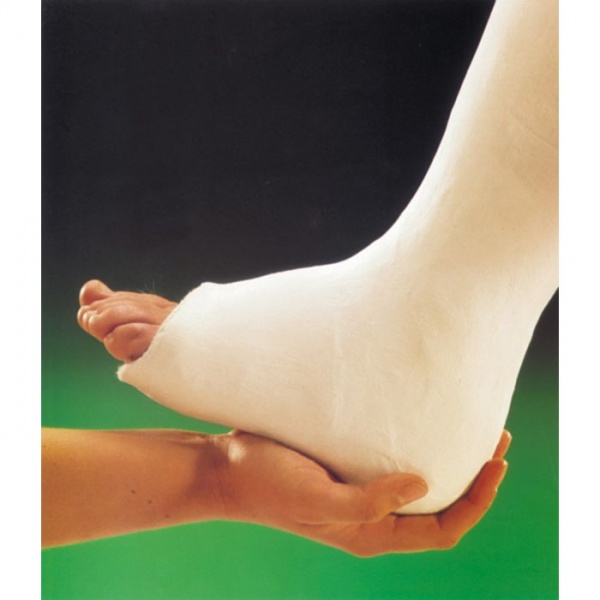
It is also used to make plaster casts to immobilize broken bones while they heal, though many modern orthopedic casts.

When dentists create dental models for people's teeth, they can be used to compare one's teeth. Espeically when starting the process of having braces.

It is used for creating art pieces by sculpting, as the speed at which the plaster sets gives the work a sense of immediacy and enables the sculptor to achieve the original idea quickly.

Are there any health concerns (toxicity, diseases) associated with any of the reactants or products in the reaction?
Reactants

Calcium Hydroxide
Food-grade calcium hydroxide is generally safe. However, if you work with industrial-grade calcium hydroxide, ingesting it can result in calcium hydroxide poisoning. This can lead to severe injury or death.
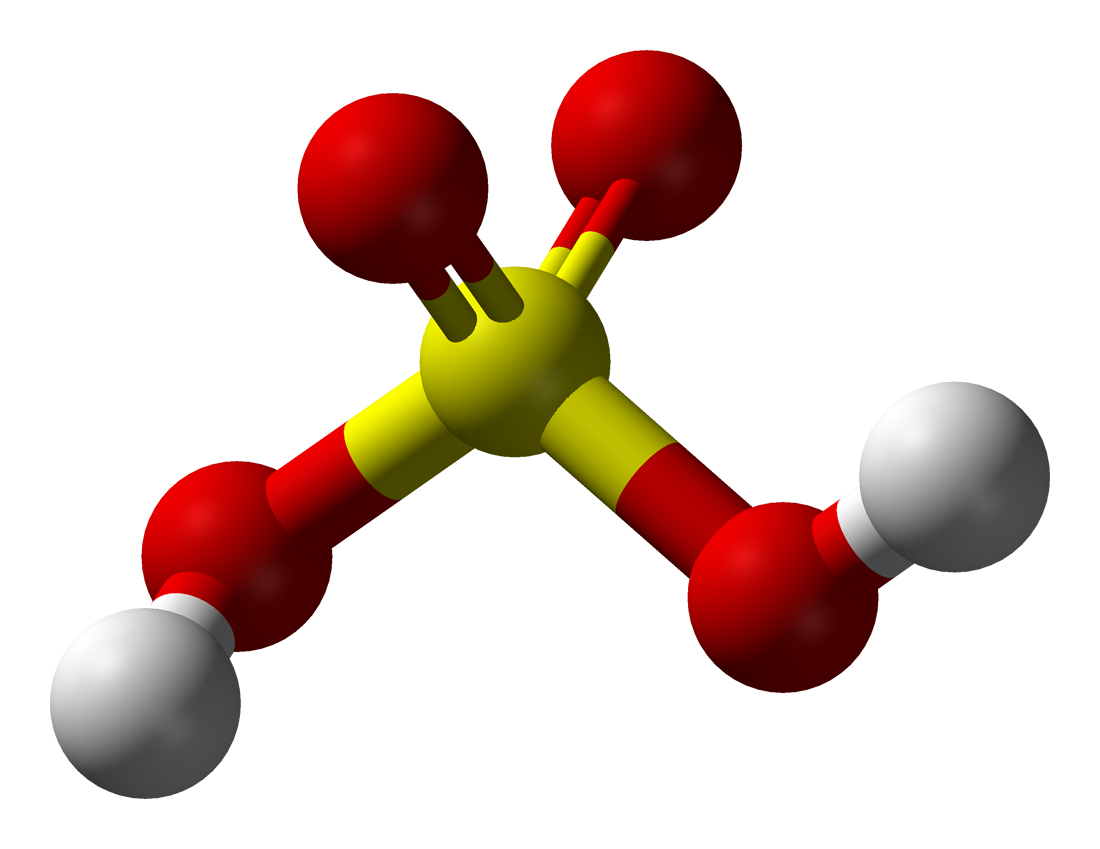
Sulfuric Acid
It is a highly corrosive chemical that is potentially explosive in concentrated form. It can cause severe skin burns, can irritate the nose and throat and cause difficulties breathing if inhaled, can burn the eyes and possibly cause blindness, and can burn holes in the stomach if swallowed.

Products

Calcium Sulfate
It is a relatively non-hazardous chemical. Repeated or prolonged contact can irritate the skin and eyes. Ingestion of large amounts of calcium sulfate can cause bronchitis, nausea, stomach upset, vomiting and diarrhea.

Water
No hazards, it's water. Don't drown?! Hahah(:
What is the product in this reaction?
Calcium Sulfate
How is it Useful to us?

Calcium sulfate is useful to us because it creates a white or colourless powder called gypsum, which is used to create soil conditioners. Soil conditioners help to loosen compacted soils as well as replenish and maintain nutrients for the plants to flourish. This is really good for the environment.

Without Calcium Sulfate, the Plaster of Paris wouldn't exist as well. This technology helps people medically and it can be used for fashion and art.
Annual Production of This Reaction?
Calcium Sulfate - Production and occurrence World production of natural gypsum is around 127 million tonnes per annum.

Are there government policies that regulate the reaction?
Yes there is one policy on the government website: https://laws-lois.justice.gc.ca/eng/regulations/c.r.c.,_c._870/page-65.html?wbdisable=true

Sulphuric acid was selected for risk evaluation because it is a substance that meets the criteria for corrosive substances as defined by OECD that, if spilled, it could immediately be harmful to humans and/or the environment.

According to the Canadian Government, it was stated that "Following the risk evaluation, Environment Canada recommends that this substance be proposed for addition to Schedule 1 of the Environmental Emergency Regulations at a threshold quantity of 3 tonnes with a minimum concentration at 0.1% and at a pH ≤ 2."
What are the costs associated with this reaction?
Depending on how many Tons you want, Calcium Sulfate ranges from $100-$125 US.
It is CDN$ 12.99 for one bag of Gypsum Soil Conditioner.