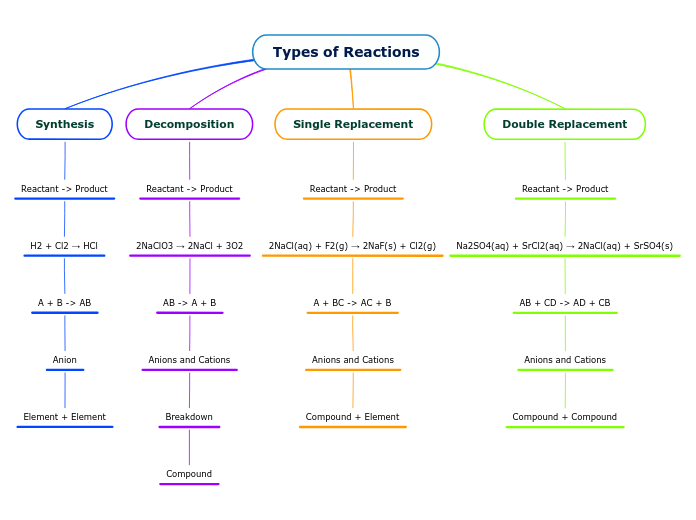
Types of Reactions
Synthesis
Reactant -> Product
H2 + Cl2 → HCl
A + B -> AB
Anion
Element + Element
Decomposition
Reactant -> Product
2NaClO3 → 2NaCl + 3O2
AB -> A + B
Anions and Cations
Breakdown
Compound
Single Replacement
Reactant -> Product
2NaCl(aq) + F2(g) → 2NaF(s) + Cl2(g)
A + BC -> AC + B
Anions and Cations
Compound + Element
Double Replacement
Reactant -> Product
Na2SO4(aq) + SrCl2(aq) → 2NaCl(aq) + SrSO4(s)
AB + CD -> AD + CB
Anions and Cations
Compound + Compound