door ALLENE WIBISONO 3 jaren geleden
313
Atmospheric Chemistry
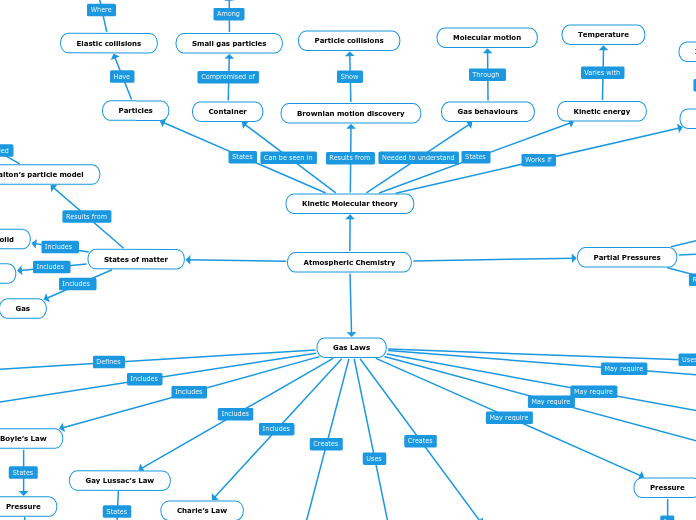
door ALLENE WIBISONO 3 jaren geleden
313

Meer zoals dit
Lots of space
Little attraction
Can flow to different places
Somewhat strong attraction
Strong attraction
The properties
The states of matter
The sum of each individual gas pressures
Ptotal = P1 + P2 + P3….
Pgas = proportion x ptotal
Not true
Conserved
Large number of particles
P1 x V1 / T1 = P2 x V2 /T2
Constant
Large quantities
Atoms or molecules
Litres
Kelvin
Gas particles
Surface
KiloPascals
Temperature and pressure
Compare different sets of experiments
Physical property
Gasses
PV=nRT
V1/T1=V2/T2
Temperature
P1/T1=P2/T2
Inverse operation
P1xV1=P2xV2
Number of particles
P1/n1=P2/n2