door Laura Frauley 4 jaren geleden
214
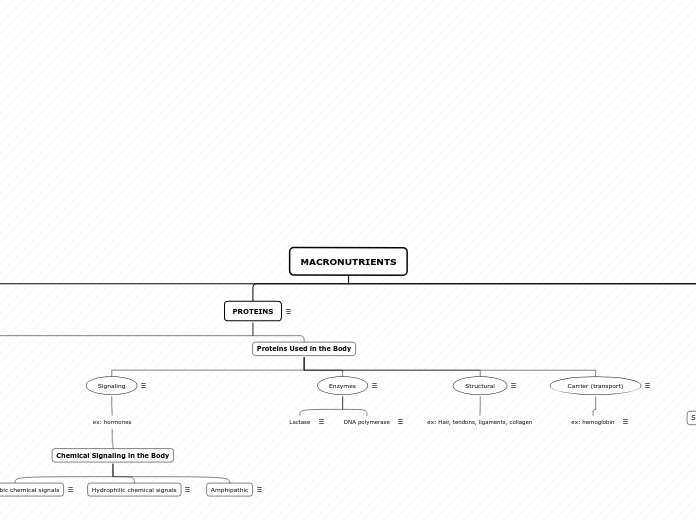
MACRONUTRIENTS
Carbohydrates are essential macronutrients that provide quick energy and serve as structural components. They are composed of carbon, hydrogen, and oxygen and come in various forms including oligosaccharides, which are short chains of monosaccharides, and polysaccharides, which are long chains of monosaccharides linked by glycosidic bonds.