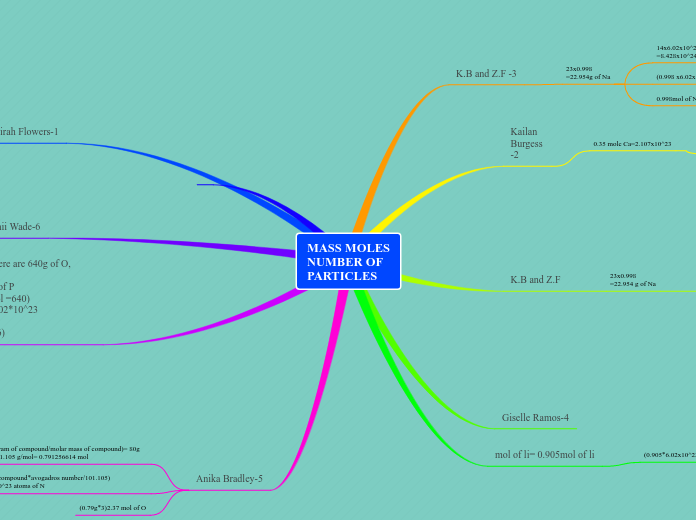
MASS MOLES NUMBER OF PARTICLES
K.B and Z.F -3
23x0.998
=22.954g of Na
14x6.02x10^23
=8.428x10^24 atoms of N
0.998x23
=22.954 mole of Na
(0.998 x6.02x10^23)= 6.00769x10^23 atoms of N
0.998mol of Na
Kailan Burgess-2
0.35 mole Ca=2.107x10^23
1 mole=6.02x10^23
0.35 mole=2.107x10^23
K.B and Z.F
23x0.998
=22.954 g of Na
14x6.02x10^23
=8.428x10^24 atoms of N
0.998x23
=22.954 mole of Na
Giselle Ramos-4
mol of li= 0.905mol of li
(0.905*6.02x10^23)=5.4481x10^23g of N
((0.905x2)x6.02x10^23)=1.0862x10^24 atoms of O
Zahirah Flowers-1
15x32/1=480g
15 mole S=480g
Dianii Wade-6
In 8mol of P2O5 there are 640g of O, 2.408*10^25
atoms of O, 16mol of P
(16g*5 atoms *8mol =640)
(8mol *5 atoms* 6.02*10^23 =2.408*10^25)
(8mol * 2atoms =16)
Anika Bradley-5
(moles=gram of compound/molar mass of compound)= 80g KNO3/101.105 g/mol= 0.791256614 mol
(gram of compound*avogadros number/101.105) 4.7558*10^23 atoms of N
(0.79g*3)2.37 mol of O