por Siti Nur Hajar Erwan 4 anos atrás
579
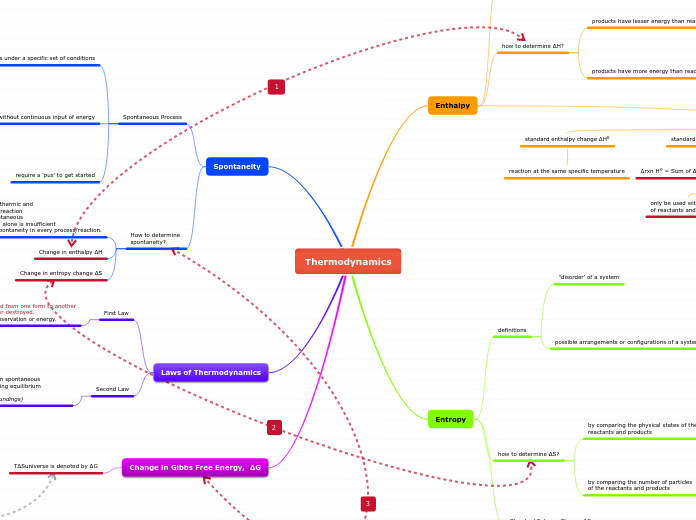
Thermodynamics
por Siti Nur Hajar Erwan 4 anos atrás
579

Mais informações
Type in the name of your subject.
spontaneous process, ∆S universe = + ve
ΔG = ∆H system - T∆S system
equilibrium process, ∆S universe = 0
Add detailed notes about each lecture, so that when the time comes to prepare for exams, you will have an easier and quicker overview.
Add a list of questions to help you recap your lecture.
Write down if there are things you would like to discuss or clarify with your teacher or colleagues in relation to this topic.
spontaneous
ball moving down a slope
non-spontaneous
water freezing at 25 °C 1 atm
Add a short description of the lecture.
Review your resource requirements and tick off the devices you will need as well as their availability. Add others, if necessary.
Add other resources:
decrease in the number of particles
increase in the number of particles
gas to solid
decrease in the number of possible arrangements
solid to gas
increase in the number of possible arrangements
Select as needed:
Examples
least number of possible outcomes
solid state
most number of possible outcomes
gaseous state
Type in all the info you would like to know about this subject. If there is something you don't know yet, no problem! You can fill in the blanks along the way.
Did your teacher present the objectives of this course? Write them down and add anything else that might help you reach these objectives.
bond forming
energy released
ΔH bond forming is -ve
bond breaking
energy is absorbed
ΔH bond breaking is +ve
Δf H° of an element & 7 diatomic elements (H2, N2, O2, F2, Cl2, Br2, l2)
Δf H° = 0 kJ/mol
one mole of compound is formed from its element in their reference states
∆rxn H° = Sum of Δf H° (product) - Sum of Δf H° (reactant)
unit of Δrxn H° is kJ
only be used with the Δf H° of reactants and products
reaction at the same specific temperature
Type in the name of your teachers and teacher assistants, plus any details you should know about them.
Heat absorbed to surroundings
endothermic reaction
+ve ΔH
Heat released to surroundings
exothermic reaction
-ve ΔH
Add details about your course.