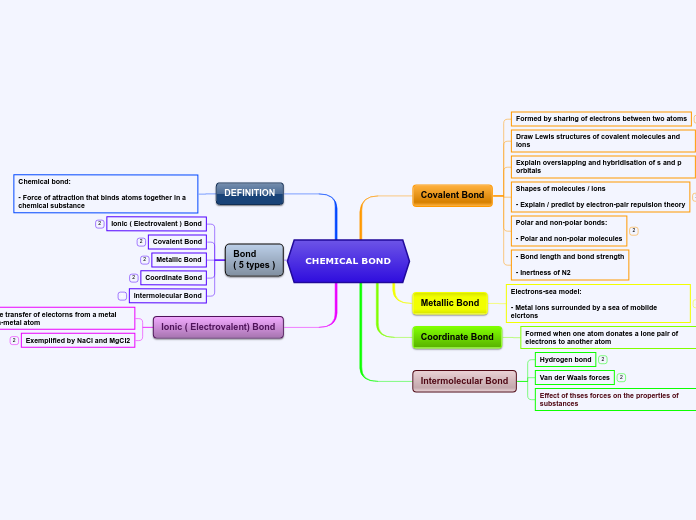
CHEMICAL BOND
Covalent Bond
Formed by sharing of electrons between two atoms
Draw Lewis structures of covalent molecules and ions
Explain overslapping and hybridisation of s and p orbitals
Shapes of molecules / ions
- Explain / predict by electron-pair repulsion theory
Polar and non-polar bonds:
- Polar and non-polar molecules
- Bond length and bond strength
- Inertness of N2
Metallic Bond
Electrons-sea model:
- Metal ions surrounded by a sea of mobilde elcrtons
Coordinate Bond
Formed when one atom donates a lone pair of electrons to another atom
Intermolecular Bond
Hydrogen bond
Van der Waals forces
Effect of thses forces on the properties of substances
DEFINITION
Chemical bond:
- Force of attraction that binds atoms together in a chemical substance
Bond
( 5 types )
Ionic ( Electrovalent ) Bond
Covalent Bond
Metallic Bond
Coordinate Bond
Intermolecular Bond
Ionic ( Electrovalent) Bond
Formed by the transfer of electorns from a metal atom to a non-metal atom
Exemplified by NaCl and MgCl2