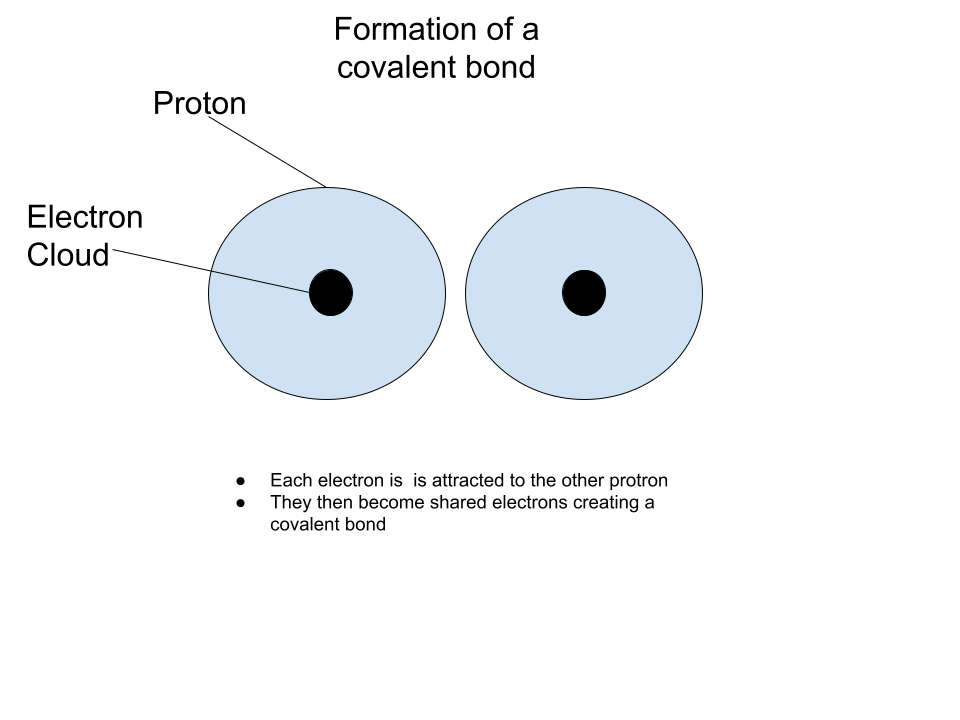
Covalent Bonds
Ion- - an atom or molecule with an electrical charge resulting from - or + of 1 or more electrons
Chemical Bonds - Allow atoms to stay close together
Ionic Bond - the transfer of electrons
Covalent Bond - Shares electrons between them
Molecule - 2 or more atoms held together
Electronegativity - measure of attraction for shared electrons
Hydrogen Bond - Weak Bond,
Polar Molecule - has an unequal distribution of charges, Ex.) Water
Reactants - The conversion of starting material
Products - The final results after the conversion
Chemical Reaction - breaking existing chemical bond and creating new ones
Distribution of Electrons
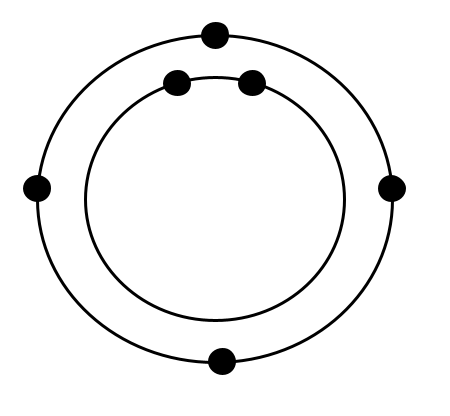
Carbon Example
Nonpolar Covalent Bonds - A covalent bond of two atoms of the same element